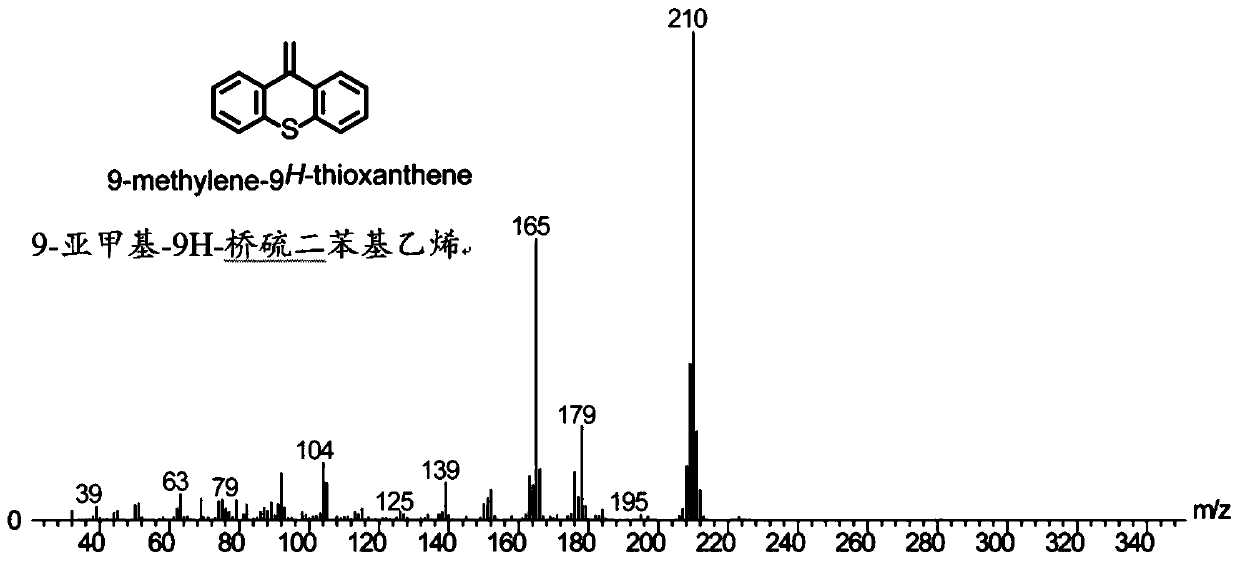
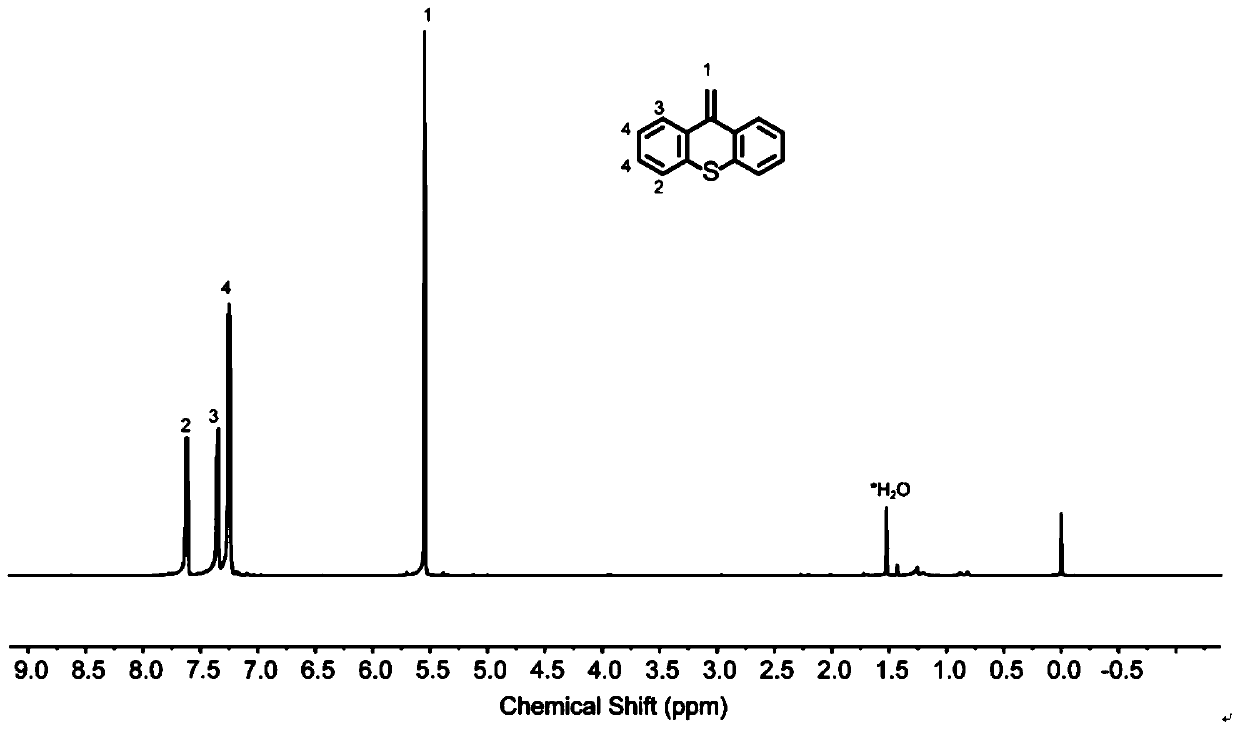
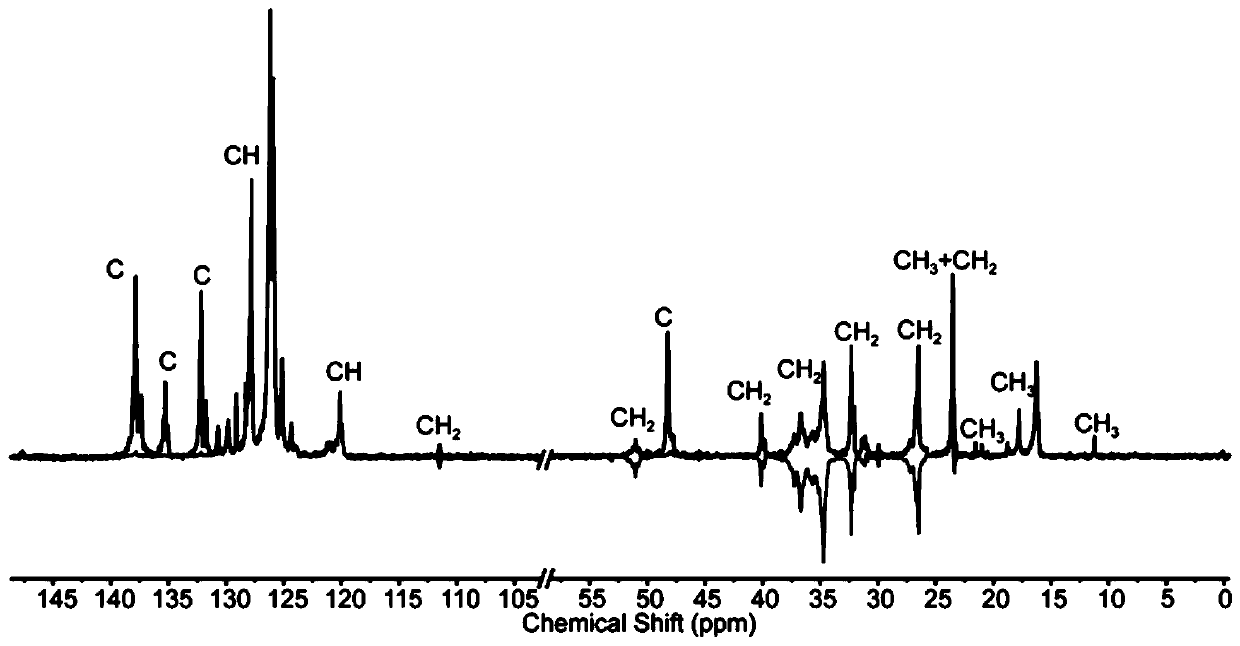
Sulfur-containing high-trans elastomers with adjustable content of trans-1,4-structure and preparation method of elastomers
A high-trans, elastomer technology, applied to a class of sulfur-containing high-trans elastomers with adjustable trans-1,4-structure content and its preparation field, which can solve the problem of limited applications and low glass transition temperature Tg , It is difficult to form alternate chain links, etc., to achieve the effect of good dynamics and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 (No. 1)
[0036] Under the protection of argon, add 260mL of solvent toluene and 1.82g of MTAE to the 1L polymerization reactor that has been dried and deoxygenated, raise the temperature to 70°C, turn on the stirring, add the initiator n-butyl lithium according to the design molecular weight of 50kg / mol, and react for 30min Add THF 0g (THF / Li=0), butadiene (1.85M toluene solution) 500mL, styrene 8.82g successively, continue the reaction for 7h, add isopropanol to terminate, and the reaction mixture is precipitated in excess anhydrous methanol , and the resulting polymer was dried in a vacuum oven to constant weight. The product structure analysis results are as follows: in terms of mass percentage, the styrene content in the terpolymer is 14.5%, the MTAE content is 3.1%, and the rest is butadiene; the number average molecular weight is 55kg / mol, and the molecular weight distribution is 1.05; Based on 100% of the total mass of dienes, the content of 1,2-po...
Embodiment 2
[0037] Embodiment 2 (No. 1)
[0038] Under the protection of argon, add 260mL of solvent toluene and 3.75g of MTAE to the 1L polymerization reactor that has been dried and deoxygenated, raise the temperature to 30°C, turn on the stirring, add the initiator n-butyllithium according to the designed molecular weight of 300kg / mol, and react for 20min Then add tetrahydrofuran THF 4.33g (THF / Li=50 equivalent), butadiene (1.85M toluene solution) 500mL, styrene 8.82g, continue reaction 10h, add isopropanol to terminate, reaction mixture is in excess anhydrous methanol The resulting polymer was dried in a vacuum oven to constant weight. The product structure analysis results are as follows: in terms of mass percentage, the styrene content in the terpolymer is 14.1%, the MTAE content is 6.2%, and the rest is butadiene; the number average molecular weight is 283kg / mol, and the molecular weight distribution is 1.04; Based on 100% of the total mass of dienes, the content of 1,2-polybutadi...
Embodiment 3
[0039] Embodiment 3 (No. 1)
[0040] Under the protection of argon, add 260mL of solvent xylene and 6.53g of MTAE to the 1L polymerization reactor that has been dried and deoxygenated, raise the temperature to 80°C, turn on the stirring, add the initiator n-butyllithium according to the design molecular weight of 500kg / mol, and react 10min; then add tetrahydrofuran THF 4.33g (THF / Li = 100 equivalents), butadiene (1.85M toluene solution) 500mL, styrene 8.82g, continue the reaction for 3h, add isopropanol to terminate, the reaction mixture in excess anhydrous The resulting polymer was precipitated in methanol and dried in a vacuum oven to constant weight. The product structure analysis results are as follows: in terms of mass percentage, the styrene content in the terpolymer is 13.6%, the MTAE content is 9.8%, and the rest is butadiene; the number average molecular weight is 487kg / mol, and the molecular weight distribution is 1.04; Based on 100% of the total mass of diene, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com