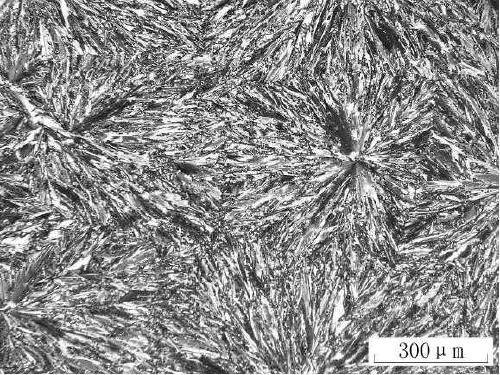
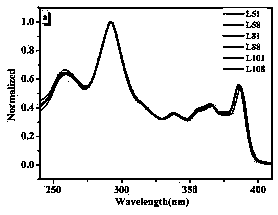
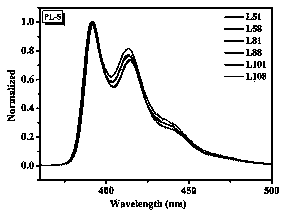
Preparation method of fluorene-containing polycyclic aromatic hydrocarbon disc-shaped liquid crystal compound
A technique for discotic liquid crystal and compound, applied in the field of discotic liquid crystal compound and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] The compound provided in this example is the compound of general formula (I), which is L51.
[0108]Add compound 3-a (100mg, 0.08mmol) and dichloromethane (40ml) into a 150ml round bottom flask, take a 25ml small beaker, add 4ml nitromethane, ferric chloride (68.14mg, 0.42mmol) , react at room temperature, track the reaction every 15 minutes, when the 3-a reaction is complete and new products are produced, add methanol to stop the reaction, extract with dichloromethane, dry the organic phase with MgSO4, filter, and spin dry the organic solvent ; Through silica gel column chromatography (eluent: V dichloromethane: V petroleum ether = 1:1), recrystallization with ethanol and ethyl acetate gave white solid L51 (93.5 mg, yield 93.8%).
[0109] 1H NMR(CDCl3, TMS, 400 MHz), δ:9.00(s, 2H, ArH), 8.52(s, 2H,ArH), 8.29(s, 2H, ArH), 8.11(s, 2H, ArH) ), 7.87(s, 4H, ArH), 4.39(t, J = 5.6Hz, 4H, OCH2), 4.29(t, J = 3.6 Hz, 12H, OCH2), 1.97-2.07(m, 16H, CH2), 1.84(s, 6H, 2CH3), 1.49-...
Embodiment 2
[0114] The compound provided in this example is the compound of general formula (I), which is L58.
[0115] Add compound 3-b (180mg, 0.13mmol) and dichloromethane (50ml) into a 150ml round bottom flask, take a 25ml small beaker, add 5ml nitromethane, ferric chloride (105.7mg, 0.65mmol) , react at room temperature, track the reaction every 15 minutes, when the 3-b reaction is complete and new products are produced, add methanol to stop the reaction, extract with dichloromethane, dry the organic phase with MgSO4, filter, and spin dry the organic solvent ; Through silica gel column chromatography (eluent: V dichloromethane: V petroleum ether = 1:1.5), recrystallization with ethanol and ethyl acetate gave white solid L58 (143.6 mg, yield 80.0%).
[0116] 1H NMR(CDCl3, TMS, 400 MHz), δ:9.00(s, 2H, ArH), 8.41(s, 2H, ArH), 8.30(s, 2H, ArH), 8.12(s, 2H, ArH) ), 7.89(s, 4H, ArH), 4.31(t, J = 6.8Hz, 16H, OCH2), 2.28(t, J = 7.2 Hz, 4H, 2CH2), 1.94-2.05(m, 16H, CH2), 1.48-1.62(m, 40H, C...
Embodiment 3
[0121] The compound provided in this example is the compound of general formula (I), which is L81.
[0122] Add compound 3-c (200mg, 0.13mmol) and dichloromethane (60ml) into a 150ml round bottom flask, take a 25ml small beaker, add 5ml nitromethane, ferric chloride (106.6mg, 0.66mmol) , react at room temperature, track the reaction every 15 minutes, when the 3-c reaction is complete and new products are produced, add methanol to stop the reaction, extract with dichloromethane, dry the organic phase with MgSO4, filter, and spin dry the organic solvent ; Through silica gel column chromatography (eluent: V dichloromethane: V petroleum ether = 1:2), recrystallization with ethanol and ethyl acetate gave white solid L81 (160 mg, yield 80.4%).
[0123] 1H NMR(CDCl3, TMS, 400 MHz), δ:9.00(s, 2H, ArH), 8.52(s, 2H,ArH), 8.30(s, 2H, ArH), 8.12(s, 2H, ArH) ), 7.88(s, 4H, ArH), 4.32(t, J = 6.4Hz, 16H, OCH2), 1.94-2.04(m, 16H, CH2), 1.84(s, 6H, 2CH3), 1.33-1.66(m , 80H,CH2), 0.91(t, J = ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com