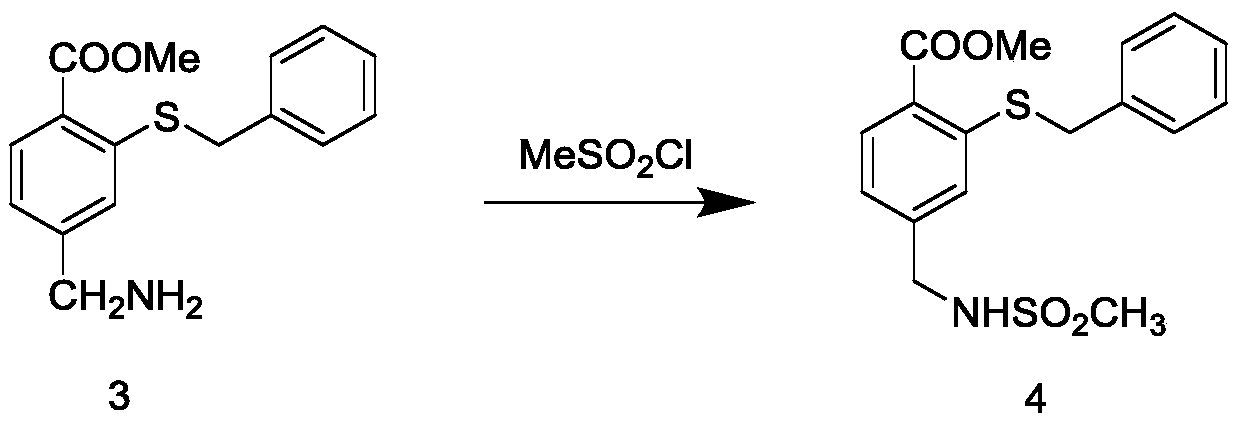
Preparation method of mesosulfuron-methyl
The technology of methyldisulfuron-methyl and the methyldisulfuron-methyl-methyl is applied in the field of preparation of methyldisulfuron-methyl, and can solve the problems such as the inability to realize cyclic application of the catalyst, the environmental pollution of potassium dichromate, the easy corrosion of production equipment, and the like, To achieve the effect of reducing the generation of high COD wastewater, cheap price, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
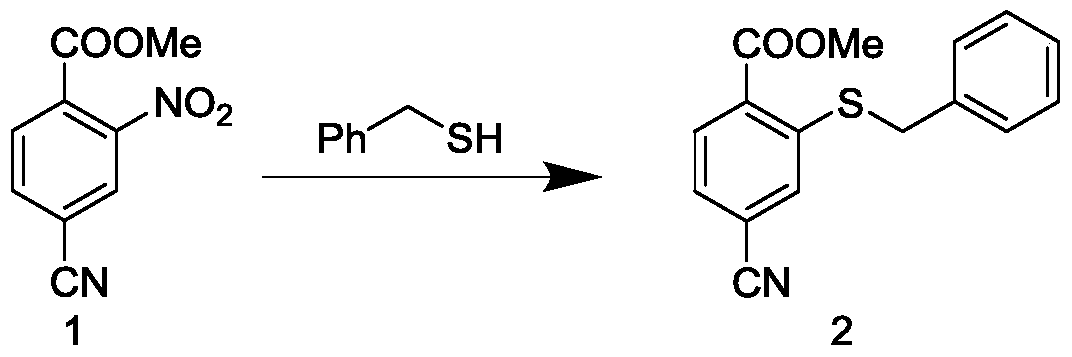
[0043] 1) Preparation of methyl 2-(benzylthio)-4-cyanobenzoate (compound 2)
[0044] 1 g of methyl 4-cyano-2-nitrobenzoate, 700 mg of benzyl mercaptan and 10 ml of DMF were added to a 50 mL flask, and the resulting solution was hydrated and cooled to 0°C. Dissolve 520mg potassium hydroxide in 2.2ml H 2 An aqueous potassium hydroxide solution was prepared in O, and the potassium hydroxide solution was slowly added dropwise to the above solution under ice-cooling. After the dropwise addition was completed at room temperature for 30 minutes, the reaction was completed, and the reaction mixture was poured into ice water, and then filtered with suction to obtain a yellow solid compound 2 with a yield of 80.1%.
[0045] The product data is: 1 H NMR (400MHz, Chloroform-d) δ8.01 (d, J = 8.0Hz, 1H), 7.57 (d, J = 1.5Hz, 1H), 7.40 (dt, J = 7.9, 1.4Hz, 3H), 7.38 –7.23(m,3H),4.15(s,2H),3.91(s,3H).
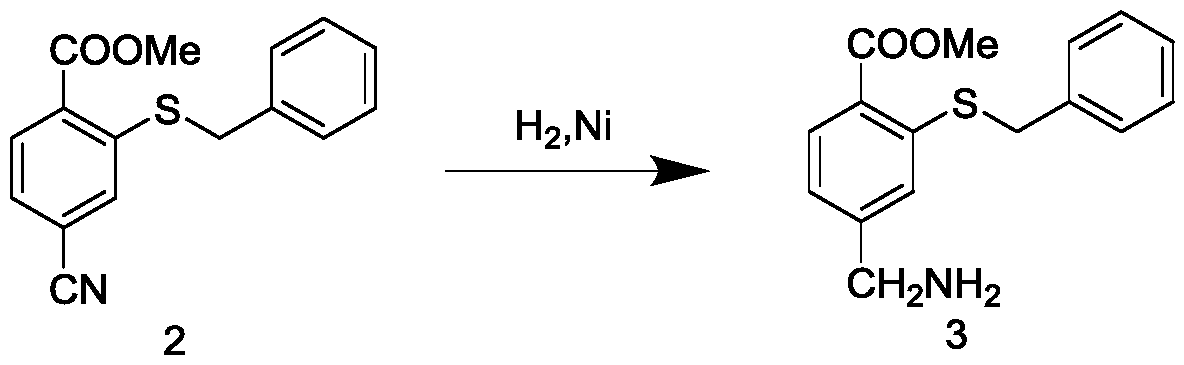
[0046] 2) Preparation of methyl 4-(aminomethyl)-2-(benzylthio)benzoate (compound 3)
...
Embodiment 2
[0059] 1) Preparation of methyl 2-(benzylthio)-4-cyanobenzoate (compound 2)
[0060] 5 g of methyl 4-cyano-2-nitrobenzoate, 3.5 g of benzyl mercaptan and 45 ml of DMF were added to a 250 mL flask, and the resulting solution was hydrated and cooled to 0°C. Dissolve 2.6g potassium hydroxide in 11ml H 2 An aqueous potassium hydroxide solution was prepared in O, and the potassium hydroxide solution was slowly added dropwise to the above solution under ice-cooling. After the dropwise addition, react at room temperature for 30 minutes, and the reaction is completed. The mixture after the reaction is poured into ice water, and then filtered with suction to obtain a yellow solid as compound 2, with a yield of more than 80%.
[0061] The product data is: 1 H NMR (400MHz, Chloroform-d) δ8.01 (d, J = 8.0Hz, 1H), 7.57 (d, J = 1.5Hz, 1H), 7.40 (dt, J = 7.9, 1.4Hz, 3H), 7.38 –7.23(m,3H),4.15(s,2H),3.91(s,3H).
[0062] 2) Preparation of methyl 4-(aminomethyl)-2-(benzylthio)benzoate (comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com