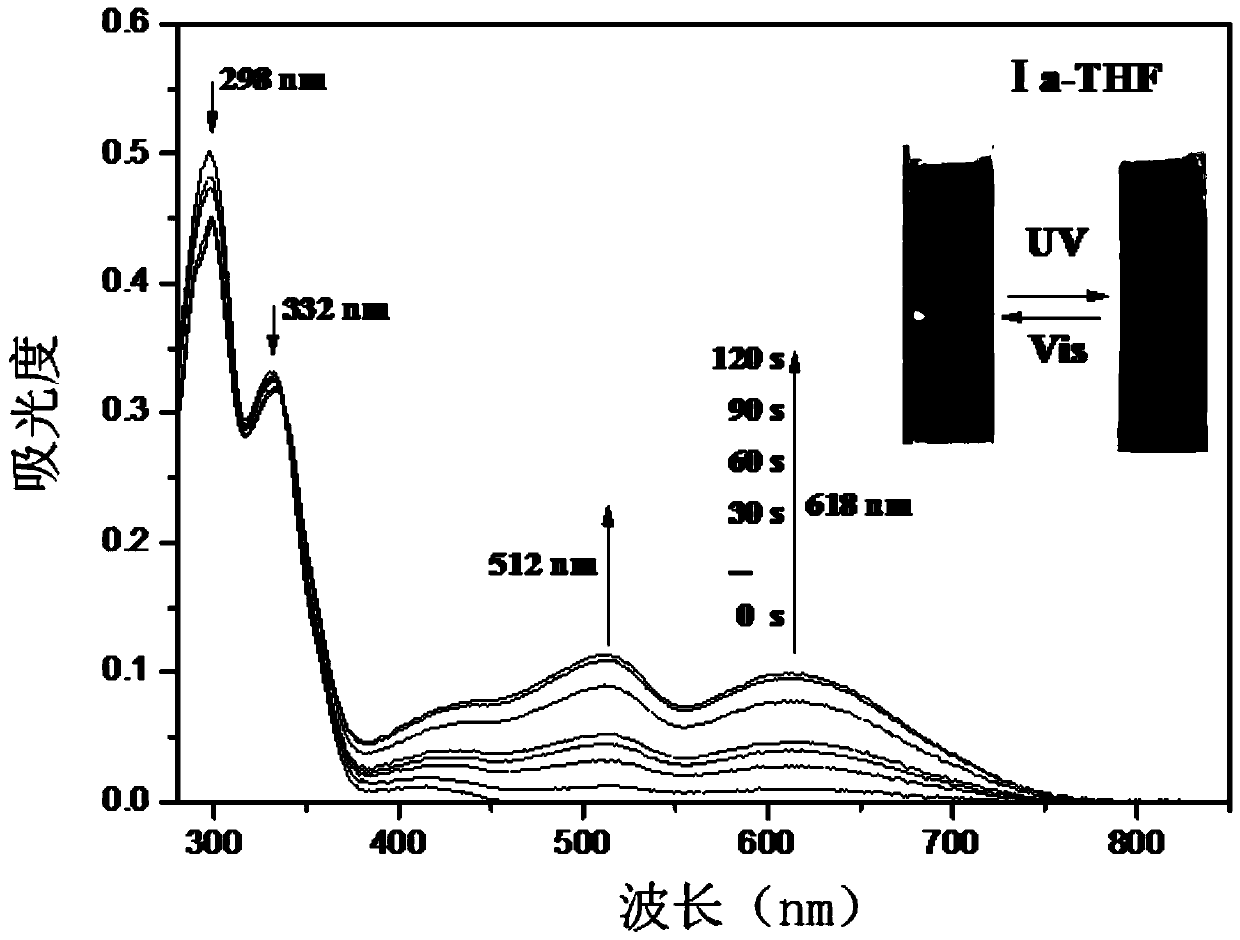
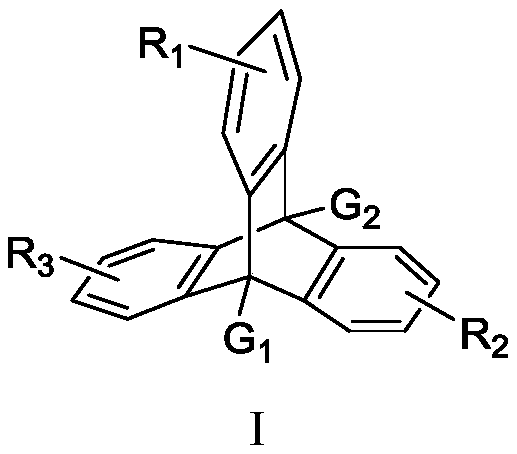
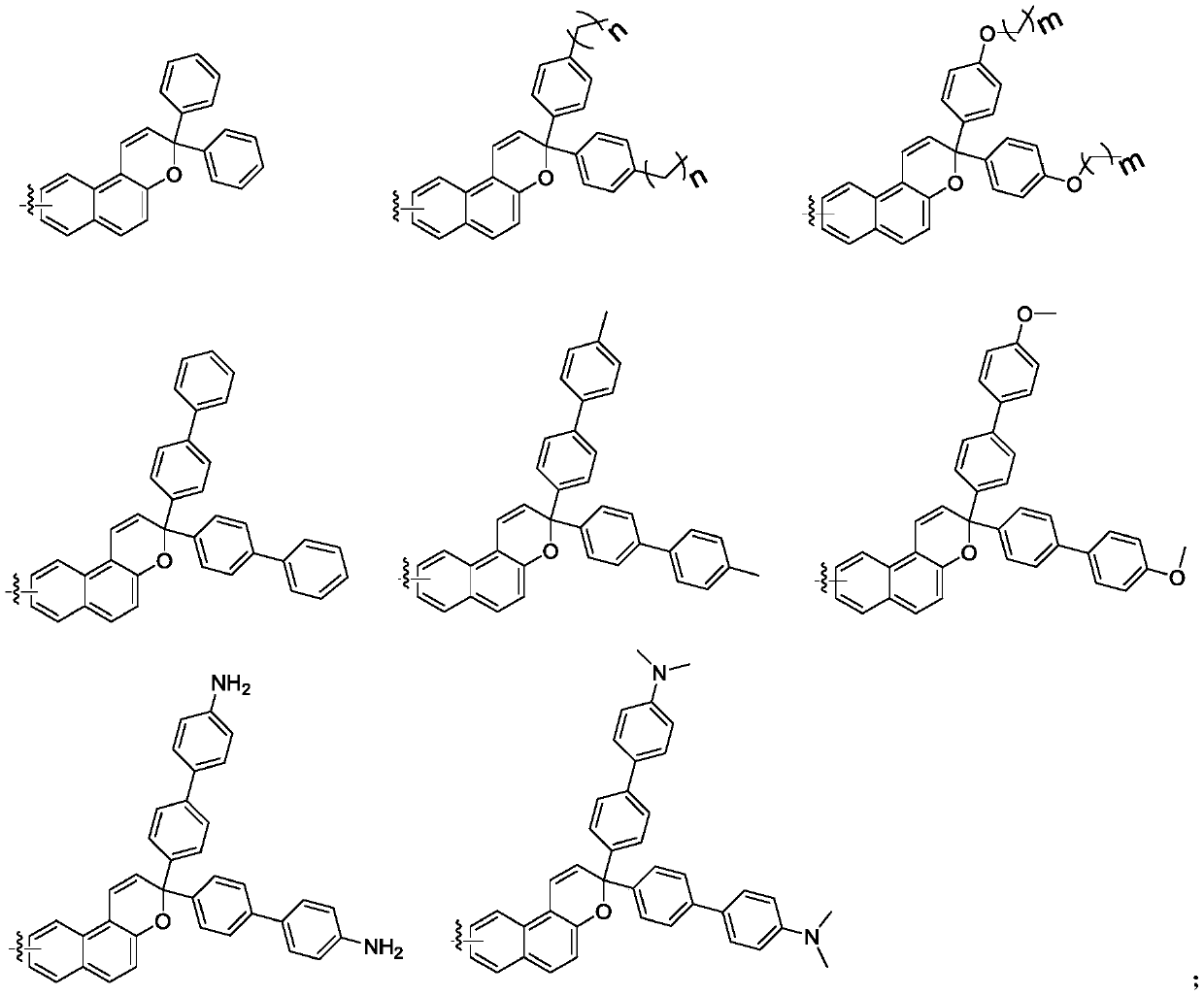
Naphthopyran branched triptycene compound, preparation method and application of naphthopyran branched triptycene compound
A technology of naphthopyran branched triptycene and naphthopyran, applied in chemical instruments and methods, organic chemistry, color-changing fluorescent materials, etc., can solve fast fading speed, low background color, lower device performance, etc. problem, achieve good reversibility, reduce planar stacking, and achieve high quantum efficiency in ring closure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The preparation method of halogenated naphthopyran compounds 2-7 was synthesized by referring to the literature: Abe, Jiro; Kato, Hirohisa; Shimizu, Takehiro; Nakagawa, Yuki. Decoloration speed adjustment method of naphthopyran compound. Jpn. Kokai Tokkyo Koho (2015), 19pp.CODEN:JKXXAF; JP2015127371.Karine Chamontin,Vladimir Lokshin,Valerie Rossollin,Andre Samat*and Robert Guglielmetti.Synthesis and Reactivity of Formyl-Substituted Photochromic 3,3-Diphenyl-[3H]-naphtho[2,1-b]pyrans .K.Chamontin et al. / Tetrahedron 55(1999)5821-5830.
[0048]7-bromo-2-hydroxynaphthalene, 8-bromo-2-hydroxynaphthalene, 1,1-bis(p-methoxyphenyl)-2-propyl-1-ol, 1-diphenylpropan-2- Alkyn-1-ol and p-toluenesulfonic acid were purchased from Ailean (Shanghai) Chemical Technology Co., Ltd.
[0049] Triptycene, concentrated nitric acid, sodium, glacial acetic acid, Raney nickel, hydrazine hydrate, tetrahydrofuran, ethyl acetate, petroleum ether, dichloromethane, sodium chloride, anhydrous sodium s...
Embodiment 1
[0064] Synthesis of Compound Ia
[0065]
[0066] Add a magneton into a 25mL two-necked flask, under argon protection and under reduced pressure, fully dry the 25mL two-necked flask with an electric oven, and dissolve 2,6-dibromotriptycene (1.0mmol, 410mg) in Into 10mL freshly distilled anhydrous tetrahydrofuran solvent, inject the above solution into the reaction flask with a syringe, stir at -78°C for 5min, then slowly add 2.5M n-BuLi (2.5mmol, 1.0mL) dropwise, and the dropwise addition is completed After continuing to react at -78°C for 1h, inject trimethyl borate (3.0mmol, 0.34mL) at one time, continue to react at -78°C for 1h, and then slowly rise to room temperature for 4h. Without treatment, the obtained three The mixture of trimethyl pterene borate (1) was directly used in the next step of Suzuki coupling reaction.
[0067] Add a magneton into a 50mL two-necked flask, weigh compound 2 (2.0mmol, 0.8g) and dissolve it in 10mL of redistilled tetrahydrofuran and add it...
Embodiment 2
[0071] Synthesis of Compound Ib
[0072]
[0073] Add a magneton into a 25mL two-necked flask, protect it with argon and under reduced pressure, fully dry the 25mL two-necked flask with an electric oven and ventilate it three times, and 2,6-dibromotriptycene (1.0mmol, 410mg) was dissolved in 10mL of freshly distilled anhydrous tetrahydrofuran solvent, and the above solution was injected into the reaction flask with a syringe, and after stirring at -78°C for 5min, 2.5M n-BuLi (2.5mmol, 1.0mL) was slowly added dropwise After the dropwise addition was completed, continue to react at -78°C for 1h, inject trimethyl borate (3.0mmol, 0.34mL) at one time, continue to react at -78°C for 1h, then slowly rise to room temperature for 4h without treatment , the obtained mixture of triptycene borate trimethyl (1) was directly used in the next step of Suzuki coupling reaction.
[0074] Add a magneton into a 50mL two-necked flask, weigh compound 3 (2.0mmol, 0.8g) and dissolve it in 10mL o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com