Composition for diagnosing hemorrhagic fever with renal syndrome, comprising seoul virus- and puumala virus- derived recombinant nucleocapsid protein and hantaan virus-derived recombinant glycoprotein, and diagnostic kit comprising same
A technology of nucleocapsid protein and hantavirus, applied in disease diagnosis, biological testing, biomaterial analysis, etc., can solve the problems of low sensitivity and specificity, low price, and low sensitivity, so as to improve diagnostic sensitivity and specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] [Example 1: Preparation of recombinant nucleocapsid protein derived from Seoul virus and Puumala virus]
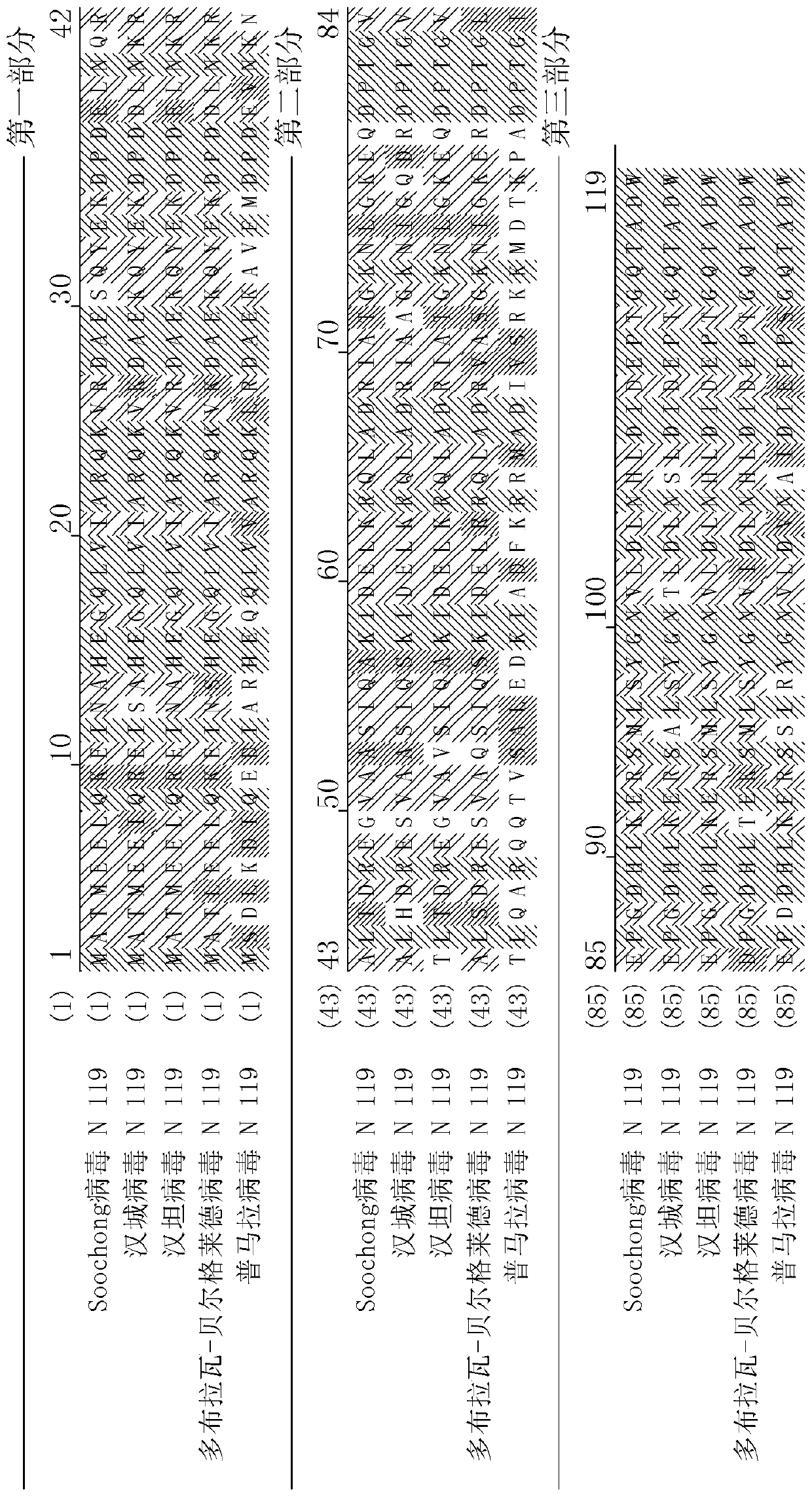
[0084] The nucleocapsid protein coding gene derived from Seoul virus (SEQ ID NO:1; 357bp; 119aa) was connected with the nucleocapsid protein coding gene derived from Puumala virus (SEQ ID NO:2; 357bp; 119aa) to synthesize the whole nucleocapsid Capsid protein gene (714bp; 238aa) (Bioneer, Korea).
[0085] The cloned nucleocapsid protein gene and the pET-32a(+) vector were completely cut into the pBHA vector with restriction enzymes EcoRI and XhoI, and after electrophoresis was performed on 1.2% agarose gel, the QIAGEN gel recovery kit (gel elution kit ) (QUAGEN Inc, USA) for recovery.
[0086] Cut the band according to the expected size on the UV transilluminator (transilluminator) and move it to a microtube. After adding 100 μl of salt solution to each net weight of 0.1 g agarose gel, place it at 37 ° C for 20 minutes to make the gel co...
Embodiment 2
[0109] [Example 2: Preparation of recombinant glycoprotein derived from Hantavirus]
[0111] Based on the base sequence of the glycoprotein gene derived from Hantavirus, the antigenic part is selected and recombined to prepare a recombinant gene composed of the base sequence of SEQ ID NO:3. Specifically, 94aa (282bp) from 414aa (1,242bp) to 506aa (1,518bp) of Hantavirus glycoprotein and 236aa (708bp) from 773aa (2,319bp) to 1,009aa (3,027bp) were combined, and the overall synthesis was 330aa (990bp) recombinant glycoprotein gene (Bioneer, Korea).
[0112] The glycoprotein gene and the pET-32a(+) vector were completely cut into the pBHA vector with restriction enzymes NdeI and XhoI, electrophoresis was performed on a 1.2% agarose gel, and the QIAGEN gel extraction kit (QUAGEN Inc., USA) for recycling.
[0113] After cutting the band according to the expected size on the UV transilluminator, pipette it into a microtube, add 100 μl of salt solution pe...
Embodiment 3
[0135] [embodiment 3: make kit]
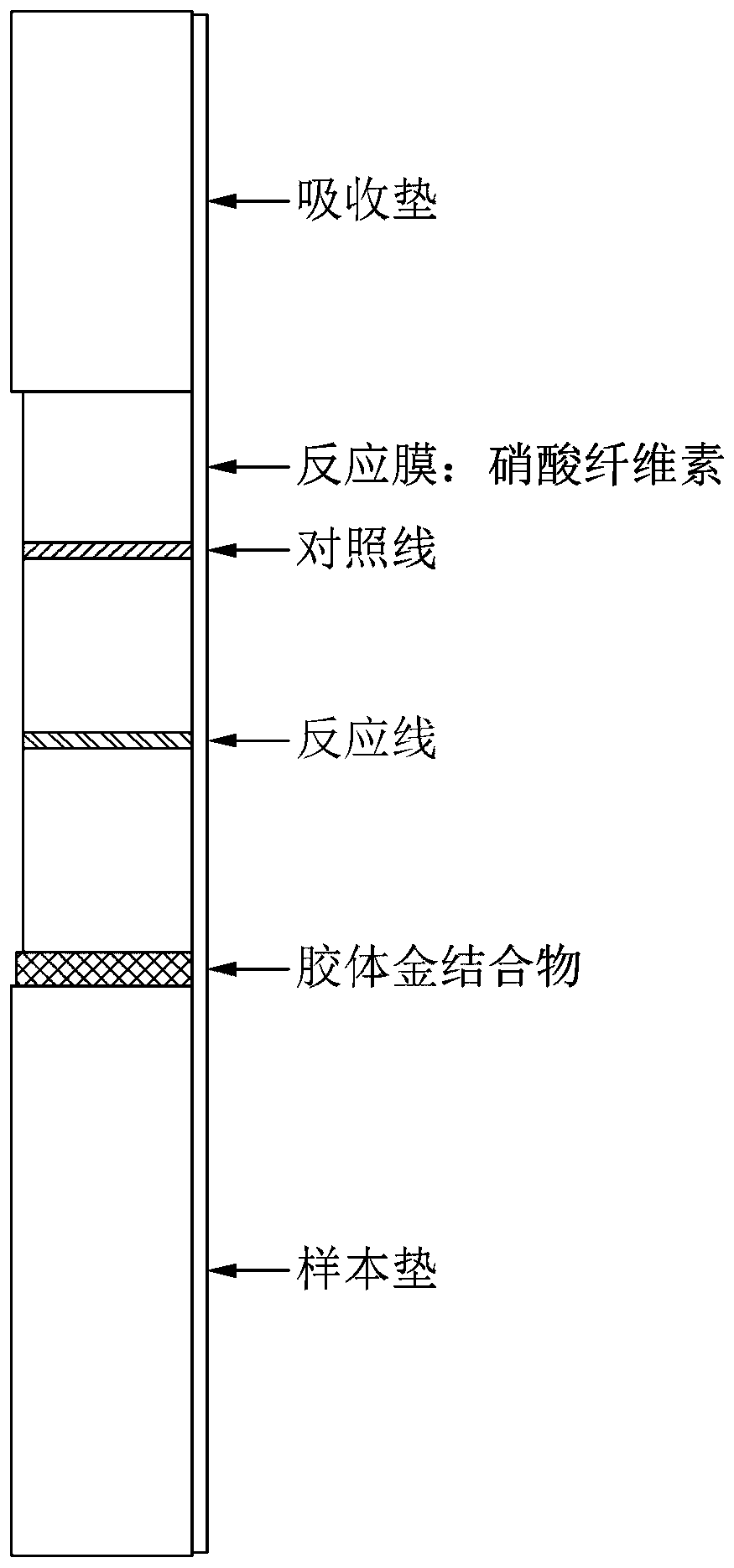
[0136] In order to confirm the effect of the renal syndrome hemorrhagic fever diagnostic agent of Seoul / Pumala virus nucleocapsid protein and Hantaan virus glycoprotein produced according to the above-mentioned Examples 1 and 2, a dispenser (dispenser) (KINEMATIC Automation) was used to produce Samples of diagnostic test strips ( figure 2 ).
[0137] Specifically, the test piece is composed of a reaction film, a sample pad, an absorbent pad, and a gold bonding pad (glass fiber). A product manufactured by Millipore was used for the reaction film, and plastic was attached to one side in order to prevent damage to the film. Three kinds of proteins were dripped into the reaction membrane.
[0138] One is to detect antibodies to the renal syndrome hemorrhagic fever virus present in the patient's serum, which will be mixed with antigenic proteins expressed from Escherichia coli or insect cells (Seoul / Pumala virus nucleocapsid protein, Hantaan vi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com