Efficient separation and culture method of fat EPCs (endothelial progenitor cells) without enveloping
A technology for endothelial progenitor cells and cells, applied in the field of obtaining endothelial progenitor cells, can solve the problems of FN and serum, complicated operation procedures, etc., and achieve the effects of clear components, low operation requirements, and reduced material costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Separation, extraction and enrichment of fat-derived EPC
[0040] 1. Configuration of complete medium: complete medium is to add respectively in IMDM serum-free medium: vascular endothelial growth factor VEGF, epidermal growth factor EGF, basic fibroblast growth factor bFGF each 10ng / mL and 5‰ ( V / V) platelet lysate.
[0041] 2. Collection of human adipocytes: collect 40ml of adult fat under aseptic conditions to a biological safety cabinet, transfer it into two 50ml centrifuge tubes on average, add 20ml of PBS respectively, mix thoroughly and centrifuge at 1000r / min for 10min, and collect the upper layer of fat Tissues were washed twice as above, and rinsed to remove interstitial fluid and blood cells in the tissues.
[0042] 3. Add 20ml of collagenase I (0.1%) to a 50ml centrifuge tube containing 20ml of fat, and place it on a shaking shaker at 37°C for 1 hour to digest until there are no obvious tissue pieces. During the digestion process, shake and mix ev...
Embodiment 2
[0048] Example 2 Differential digestion and enrichment of EPC
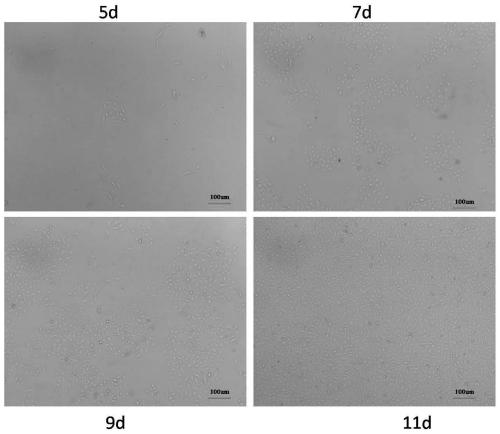
[0049] According to the aforementioned research results, the primary EPCs obtained from the 8h group were thus enriched to obtain high-purity primary EPCs for cell differential attachment time.
[0050] For the EPC cells after secondary attachment in each group in Example 1, when the cobblestone-like cell colony confluence reached ≥80%, after slowly washing the cells with PBS, use 0.1% (m / v) trypsin at 37°C Digested for 1 minute, found scattered non-clonal elongated spindle-shaped mesenchymal cells curled off, while EPC clones showed no signs of floating, rinsed with normal saline to remove, then added the same amount of trypsin to continue digestion for 2 minutes, until the cobblestone-like cells shrank After being rounded, the cells were collected by centrifugation to obtain enriched primary EPCs.
[0051] Here, the differential digestion separation method is used to enrich the EPCs of cultured adipose tissue. ...
Embodiment 3
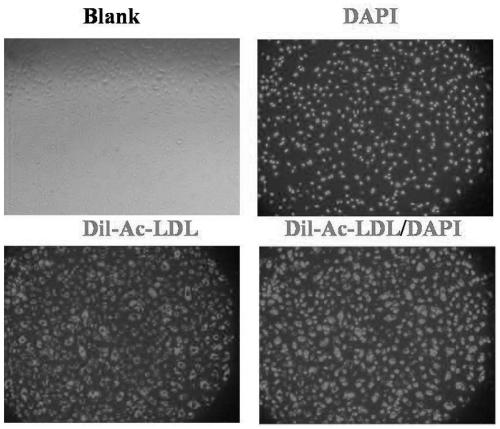
[0052] Example 3 Continuous expansion capability and cell performance verification of EPC
[0053] The primary cells obtained in Example 2 were continuously expanded in vitro using complete medium, including the following specific operations:
[0054] Cell density according to 1*10 4 / cm 2 Inoculate into the culture plate T25 (or T75), and the cell fusion rate reaches 80%-90% about every three days. After washing with PBS, directly digest with 0.1% trypsin for 2 minutes to amplify. The amplification ratio is 1:3-1 : Between 4.
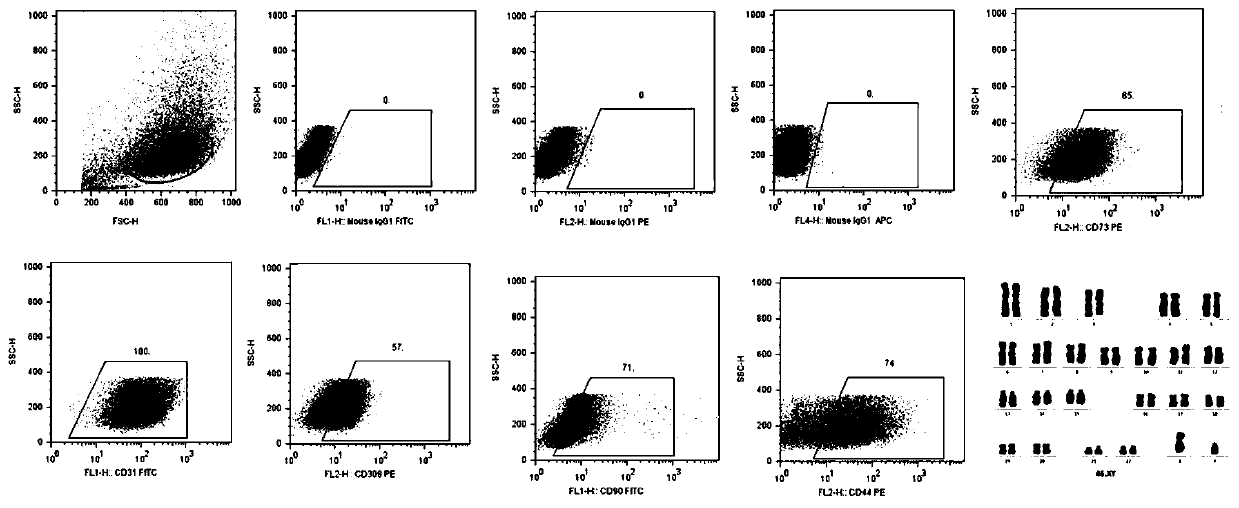
[0055] In order to further identify the purity of the cells obtained in the different attachment time groups, flow cytometric detection was performed on the first-generation EPCs isolated and obtained in different attachment time groups (results are shown in Table 1). It was found that the endothelial cell surface marker CD31 was highly expressed in the EPCs isolated from the 4h, 8h and 12h groups, and the EPC-specific marker VEGFR-2 was also expres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com