Recombinant influenza virus rescue method and application of the same in tumor therapy
A recombinant virus and anti-tumor technology, applied in the biological field, can solve the problems of complex tumor mechanism and ineffective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Embodiment 1, the rescue of recombinant influenza virus
[0093] 1. Construction of recombinant vector
[0094] The recombinant vector shown in sequence 2 is named pHW-PB1-HUPD1, the DNA molecule shown in the 887-3187 position of the sequence 2 is the PB1 gene; the sequence shown in the 3188-3253 position of the sequence 2 is the PTV-1 2A sequence ; The sequence shown in No. 3254-3313 of Sequence 2 is a signal peptide sequence; the sequence shown in No. 3314-3525 of Sequence 2 is a HUPD1 (HEAVY CHAIN) sequence; the sequence shown in No. 3526-3689 of Sequence 2 is PB1- PS packaging sequence; keep other sequences of the vector pHW2000 (sequence 1) unchanged to obtain a recombinant vector, which is named pHW-PB1-HUPD1. The recombinant vector is a vector obtained by inserting the DNA fragment (fusion gene A) shown in sequence 2 887-3689 between the BsmbI restriction sites of the pHW2000 vector, expressing PB1 and human PD1 heavy chain.
[0095] The recombinant vector show...
Embodiment 2
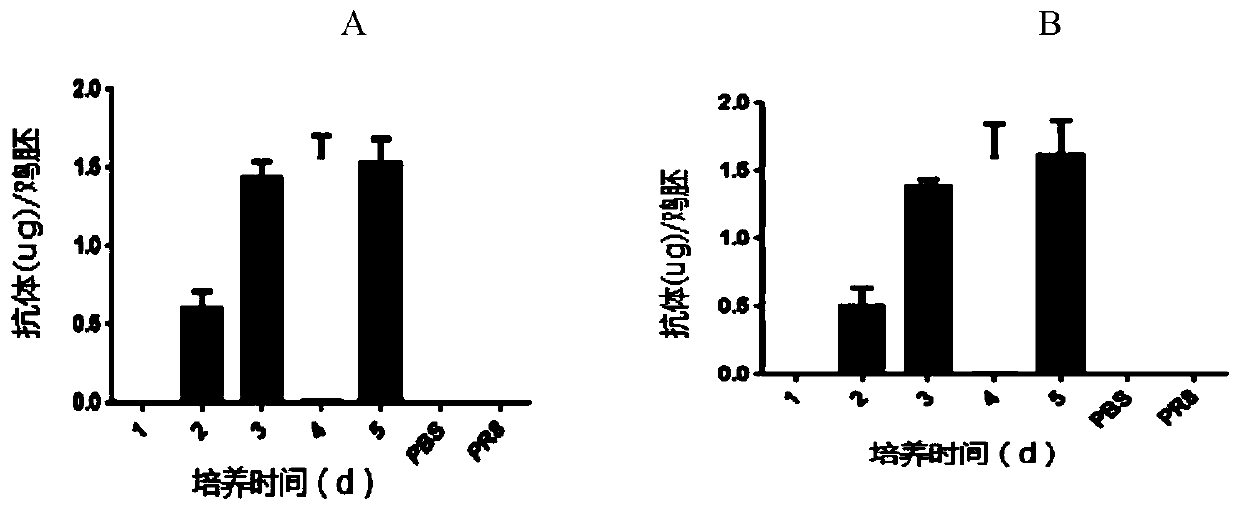
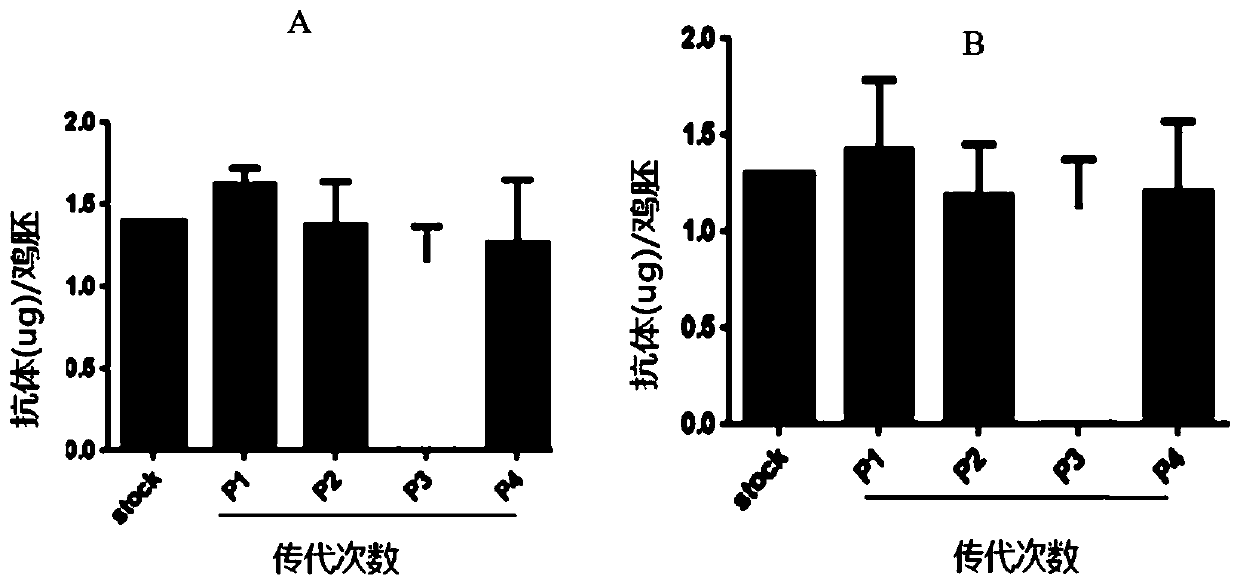
[0108] Example 2. Evaluation of the kinetics of antibody production during FLU-HUPD1 and FLU-HUPDL1 infection
[0109] Chicken embryos were inoculated with the recombinant influenza virus FLU-HUPD1 and FLU-HUPDL1 (-10PFUs) prepared in Example 1, respectively, and three 9-day-old SPF chicken embryos were selected at each time point (purchased from: Beijing Boehringer Ingel Hanweitong Biology Technology Co., Ltd.), set up PR8 (purchased from China Center for Disease Control and Prevention) control at the same time, inoculated chicken embryos with PR8 (-10PFUs), selected 3 chicken embryos at each time point, set up PBS control at the same time, and measured urine by ELISA method. The amount of anti-CTLA4 antibody present in the capsule.
[0110] The result is as figure 1 As shown, it can be seen that antibodies can be detected 2 days after inoculation with FLU-HUPD1 and FLU-HUPDL1, and reach a peak at 4-5 days. Neither the PR8 group nor the PBS group detected antibodies on the t...
Embodiment 3
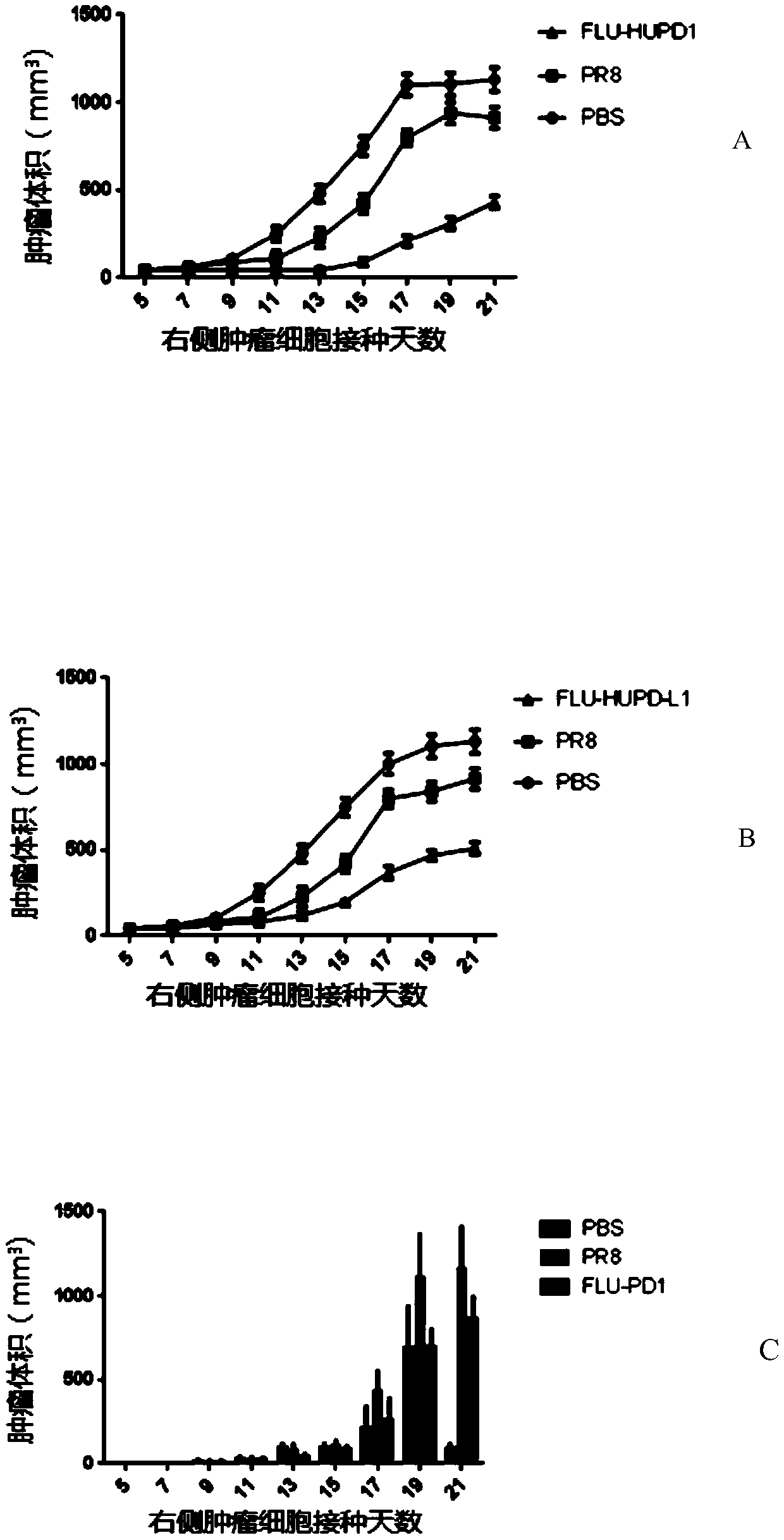
[0113] Example 3 In vivo inhibitory effect of recombinant influenza virus FLU-HUPD1 and FLU-HUPDL1 on melanoma
[0114] FLU-HUPD1 and FLU-HUPDL1 prepared in Example 1 were washed with PBS (weighed NaCl8g, KCl0.2g, NaCl 2 HPO 4 12H 2 O 3.63g, KH 2 PO 4 0.24g, dissolved in 900ml double-distilled water, adjusted the pH value to 7.4 with hydrochloric acid, added water to make up to 1L, stored at room temperature for future use) and resuspended to obtain a titer of 5.5×10 5 pfu / 100 μl of FLU-HUPD1 suspension and a titer of 5.5 × 10 5 pfu / 100 μl of FLU-HUPDL1 suspension;
[0115] Influenza virus strain A / PR / 8 / 34 (purchased from the Chinese Center for Disease Control and Prevention) was resuspended in PBS to obtain a titer of 5.5×10 5 pfu / 100 μl of influenza virus strain A / PR / 8 / 34 suspension.
[0116] 1. Preparation of mouse subcutaneous tumor model
[0117] Human CD34 on NSG mice + Cell-reconstructed humanized immune system mice (provided by Weitongda Biotechnology Co., Ltd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com