Valsartan amlodipine tablet and preparation method thereof
A technology of valsartan and amlodipine tablets and tablet cores, which is applied in the field of medicine, can solve the problems of poor content uniformity, poor dissolution of valsartan and amlodipine tablets, and low yield, and achieve high uniformity and increase drug yield. Effect of dissolution and water solubility improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
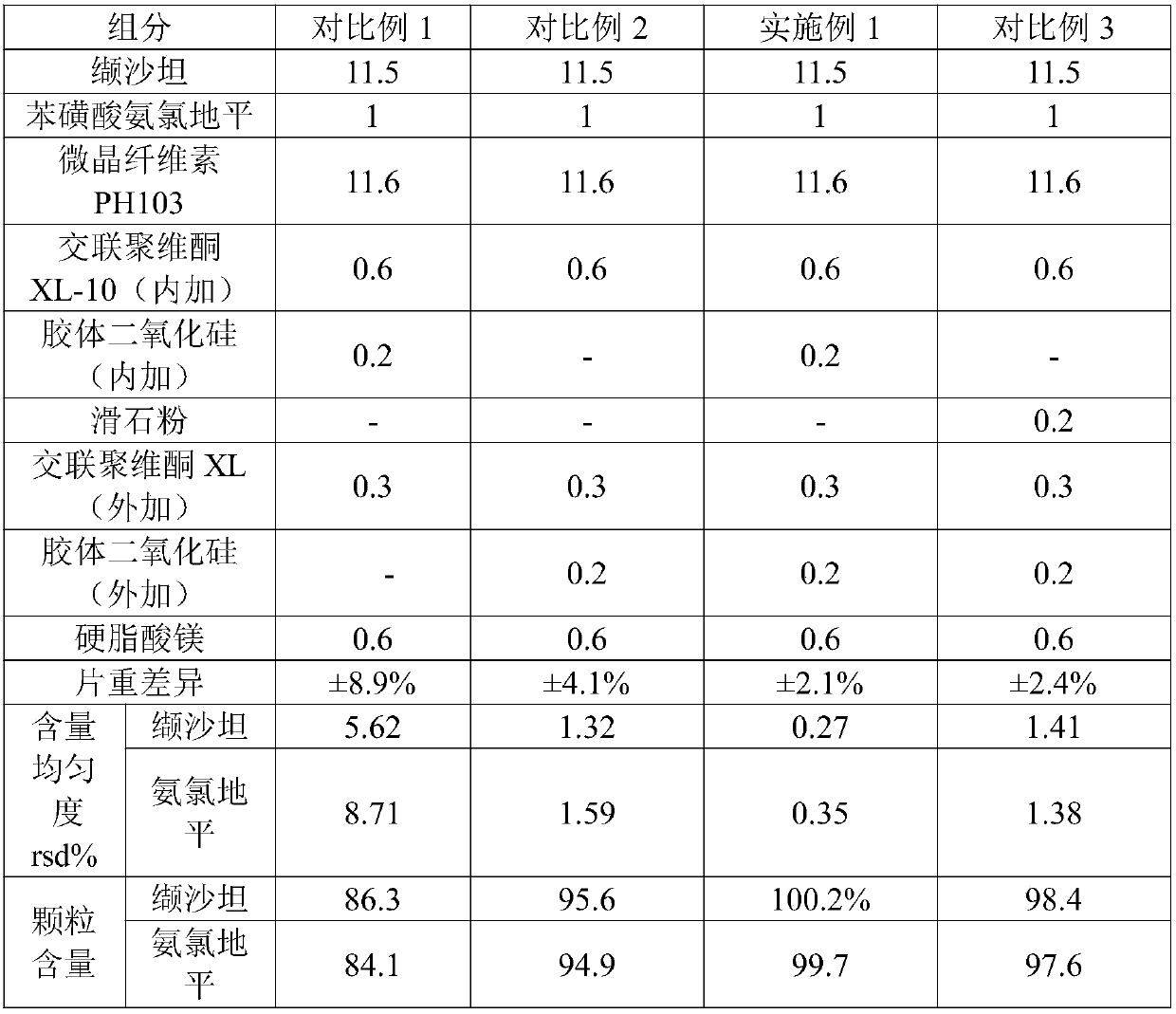
[0032] A kind of valsartan amlodipine tablet, its composition and the content of each composition are as shown in table 1 below. Its preparation method is as follows:
[0033] (1) mixing and pulverizing valsartan and colloidal silicon dioxide to obtain micronized valsartan;
[0034] (2) Amlodipine besylate and microcrystalline cellulose PH103 are uniformly mixed by equal increment method to obtain premix 1;
[0035] (3) Mix the micronized valsartan and crospovidone XL-10 (internal addition) obtained in step (1) with the premix 1 obtained in step (2) and put them into a dry granulator to prepare Get the core;
[0036] (4) The tablet core prepared in step (3), crospovidone XL (additional), magnesium stearate and colloidal silicon dioxide (additional) are mixed evenly, and valsartan amlodipine is obtained after tableting piece.
[0037] The equal incremental method in step (2) is to first mix 1 part of amlodipine besylate with 1 part of microcrystalline cellulose PH103 to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com