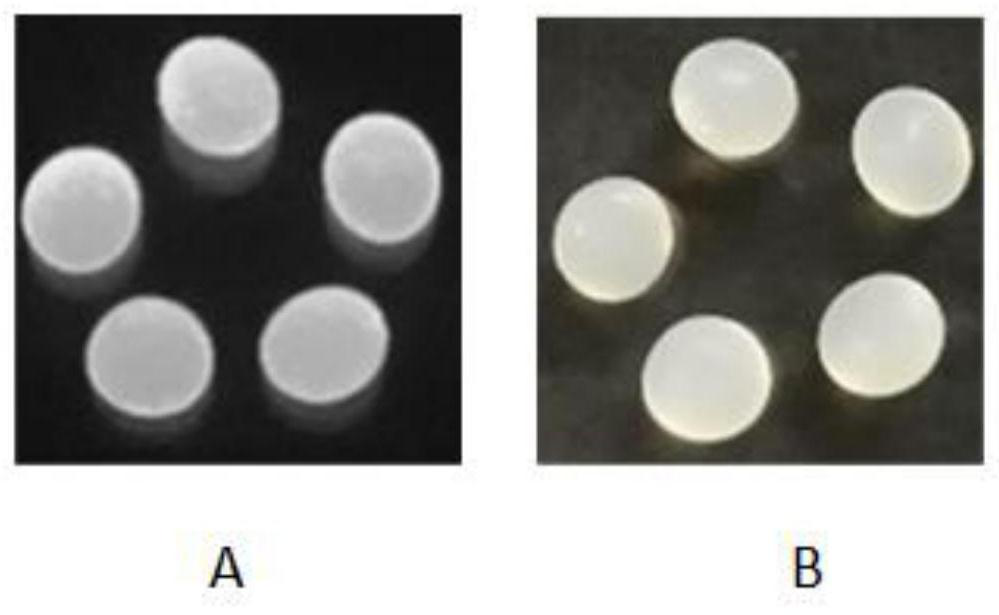
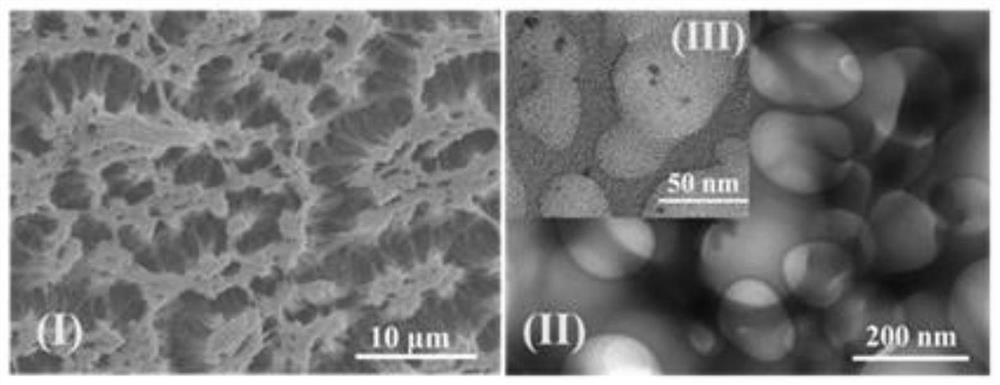
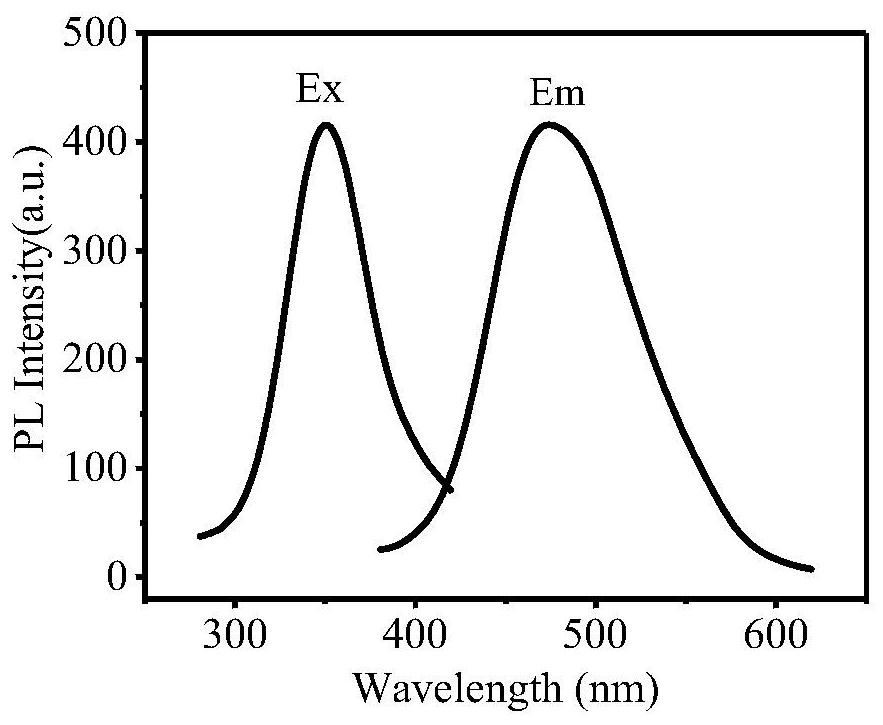
Preparation method and application of cellulose fluorescent spheres
A cellulose and microcrystalline cellulose technology, applied in the field of preparation of fluorescent carbon nanomaterials, can solve the problems of poor water solubility, limited application, poor stability, etc., and achieve the effects of good selectivity, favorable filtration separation, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation method of cellulose fluorescent ball, it comprises the following steps:
[0041] Step 1: Dissolve 0.7 g of microcrystalline cellulose powder with a molecular weight of 30,000 in 4.3 g of 1-butyl-3-methylimidazolium chloride solution, heat to 100 ° C, stir for 7 hours, and then add N, N-di Methylformamide solution 5.0g was used as a co-solvent, stirred to make the mixed solution into a homogeneous solution, and then the homogeneous solution was dripped into 1-butanol 30mL with a syringe to form round cellulose balls, while stirring 1-butyl-3-methylimidazolium chloride and N,N-dimethylformamide are removed from the cellulose balls, and then washed successively with acetone and deionized water to obtain the cellulose balls;
[0042] Step 2: 30mg of 2,2,6,6-tetramethylpiperidine oxide, 22mg of NaClO 2 and 150 mg of NaClO were dissolved in 20 mL of buffer solution, the pH of the buffer solution was 6.8, then 80 mg of wet cellulose balls were added, and react...
Embodiment 2
[0045] The preparation method of cellulose fluorescent ball, it comprises the following steps:
[0046] Step 1: Dissolve 5 g of microcrystalline cellulose powder with a molecular weight of 36,000 in 25 g of 1-butyl-3-methylimidazolium chloride solution, heat to 120°C, stir for 10 hours, and then add N,N-dimethyl Formamide solution 30g is used as a co-solvent, stir to make the mixed solution into a homogeneous solution, and then use a syringe to drop the homogeneous solution into 1-butanol 100mL to form round cellulose balls, and at the same time make the 1-butanol Butyl-3-methylimidazolium chloride and N,N-dimethylformamide are removed from the cellulose balls, and then washed successively with acetone and deionized water to obtain the cellulose balls;
[0047] Step 2: 30mg of 2,2,6,6-tetramethylpiperidine oxide, 25mg of NaClO 2 and 200 mg of NaClO were dissolved in 20 mL of buffer solution, the pH of the buffer solution was 7.0, then 100 mg of wet cellulose balls were added,...
Embodiment 3
[0050] The preparation method of cellulose fluorescent balls comprises the following steps: the molecular weight of the raw material microcrystalline cellulose used for the cellulose balls is 20,000-36,000.
[0051] Step 1: Dissolve 8 g of microcrystalline cellulose powder with a molecular weight of 20,000 in 45 g of 1-butyl-3-methylimidazolium chloride solution, heat to 110°C, stir for 7 hours, and then add N,N-dimethyl 60g of formamide solution is used as a co-solvent, stir to make the mixed solution into a homogeneous solution, then use a syringe to drop the homogeneous solution into 110mL of 1-butanol to form round cellulose balls, and at the same time make the 1-butanol Butyl-3-methylimidazolium chloride and N,N-dimethylformamide are removed from the cellulose balls, then washed with acetone and deionized water successively to obtain the cellulose balls, and then stored in deionized water for subsequent use;
[0052] Step 2: 30mg of 2,2,6,6-tetramethylpiperidine oxide, 20...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com