Supported catalyst and preparation method thereof
A supported catalyst and carrier technology, which is applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of low rate and low catalytic performance, shorten the action distance, and improve metal dispersion. , good synergistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A method for preparing a supported catalyst according to an embodiment of the present invention includes the following steps: mixing a solution containing noble metal ions with indium oxyhydroxide and adding a reducing agent for reduction reaction to obtain the above-mentioned supported catalyst.
[0030] In one specific example, the reducing agents are sodium borohydride and sodium hydroxide. Optionally, the reducing agent is a mixed solution of sodium borohydride and sodium hydroxide, wherein the concentrations of sodium borohydride and sodium hydroxide are both 0.1mol / L to 0.2mol / L, and the concentration of the solution containing noble metal ions is 1mmol / L L~5mmol / L, the reduction reaction time is 30min~60min. It can be understood that the selection of the reducing agent is not limited thereto, and other reducing agents capable of reducing Pt ions can be selected as required.
[0031] Optionally, when the noble metal is platinum, the solute in the solution contain...
Embodiment 1
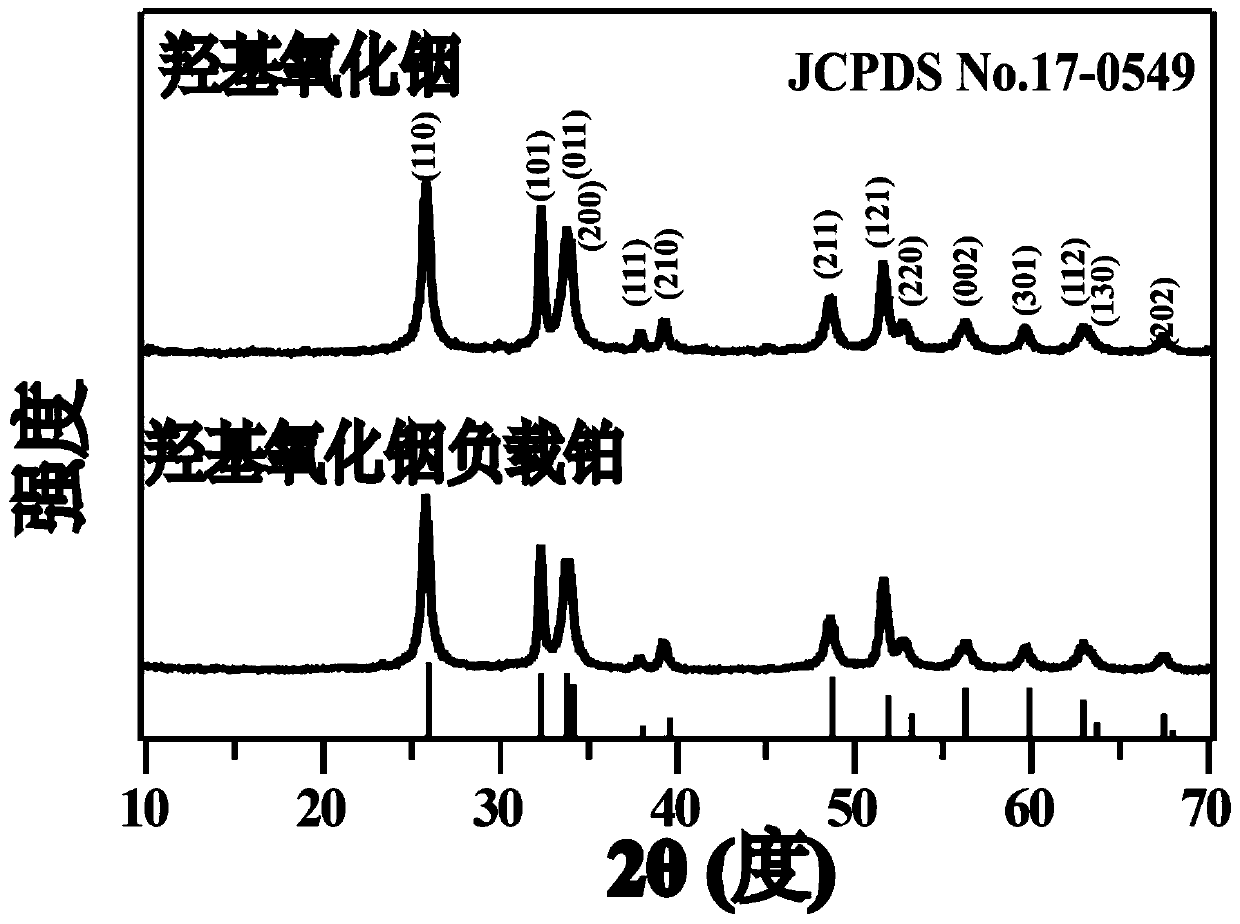
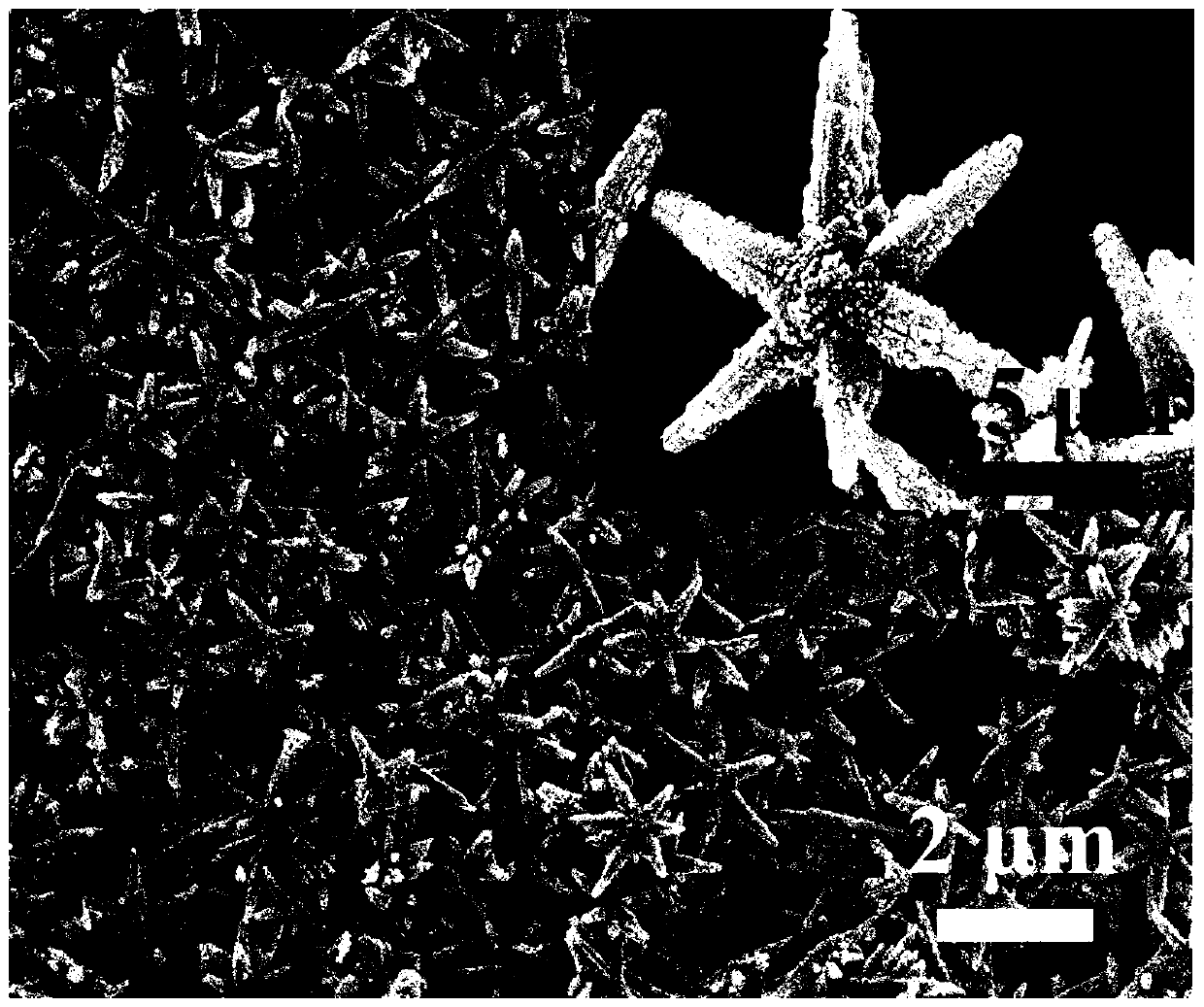
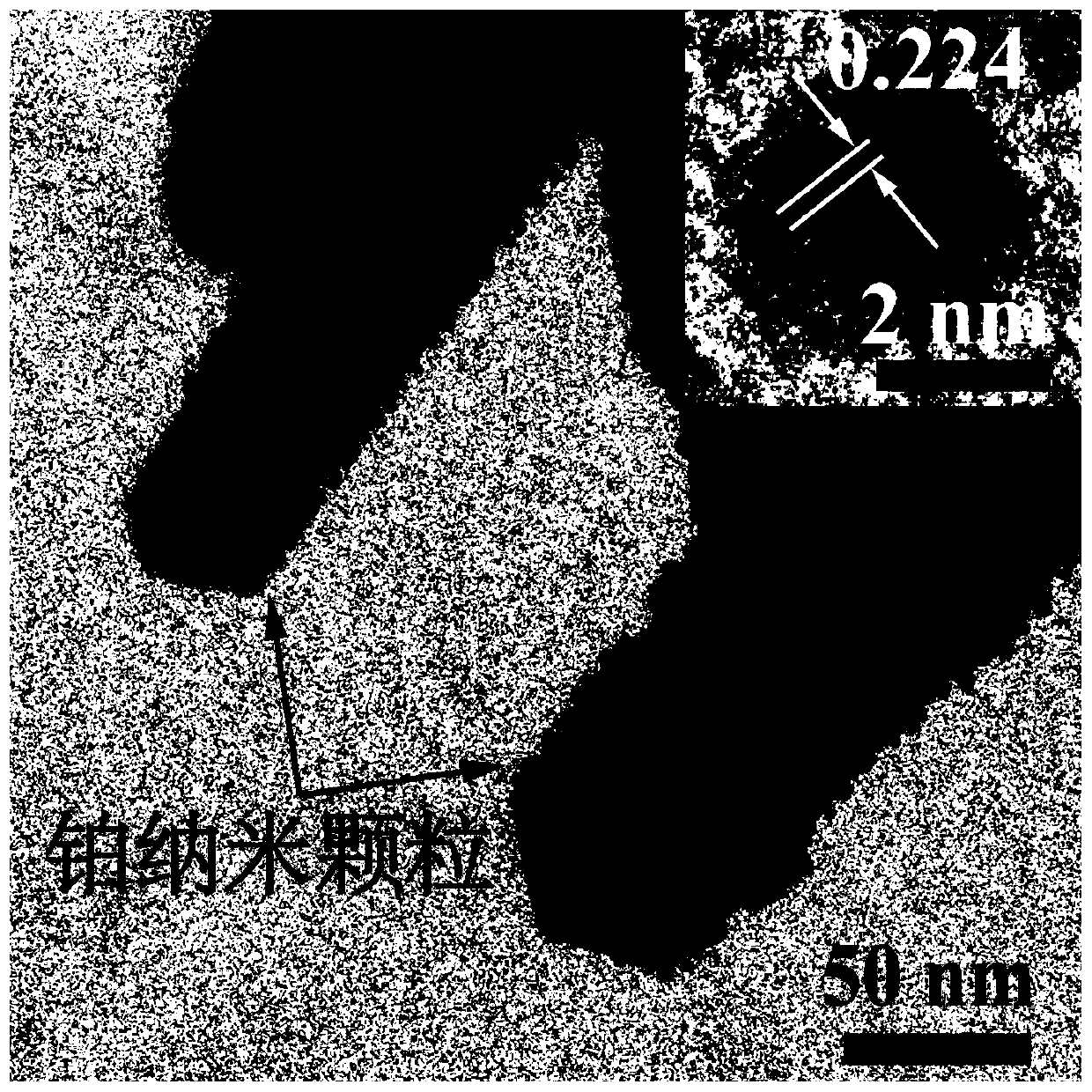
[0042] Weigh 0.7g of indium chloride tetrahydrate and disperse it in 12mL of deionized water, add concentrated hydrochloric acid drop by drop under vigorous stirring to dissolve completely, then add 2g of sodium acetate trihydrate and 48mL of ethylene glycol, continue stirring for 1 hour, and then put In the vinyl fluoride reaction kettle, heat it in an oven to 200°C and keep it warm for 24h, cool to room temperature with the oven, pour out the solution from the reaction kettle, wash the obtained product with deionized water and ethanol three times alternately, and place the sample in a 60°C oven drying in medium to obtain carrier indium oxyhydroxide. Weigh 1g of indium oxyhydroxide and add 26mL of chloroplatinic acid solution with a concentration of 2mmol / L and stir for 30min, then add 4mL of a mixture of sodium borohydride and sodium hydroxide with a concentration of 0.1mol / L, and continue stirring for 60min to dissolve the product Washed six times with water and dried in an...
Embodiment 2
[0045] Weigh 0.9g of indium nitrate monohydrate and disperse it in 12mL of deionized water, add concentrated nitric acid drop by drop under vigorous stirring to dissolve completely, then add 2.5g of sodium acetate trihydrate and 60mL of ethyl acetate, continue stirring for 1h, and then put In a vinyl fluoride reaction kettle, heat it in an oven to 180°C and keep it warm for 48 hours, then cool it to room temperature with the oven, pour out the solution from the reaction kettle, wash the obtained product three times alternately with deionized water and ethanol, and place the sample in a 60°C oven drying in medium to obtain carrier indium oxyhydroxide. Weigh 1g of indium oxyhydroxide and add 26mL of chloroplatinic acid solution with a concentration of 1mmol / L and stir for 30min, then add 2mL of a mixture of sodium borohydride and sodium hydroxide with a concentration of 0.2mol / L, and continue to stir for 30min to dissolve the product Washed six times with water and dried in an o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com