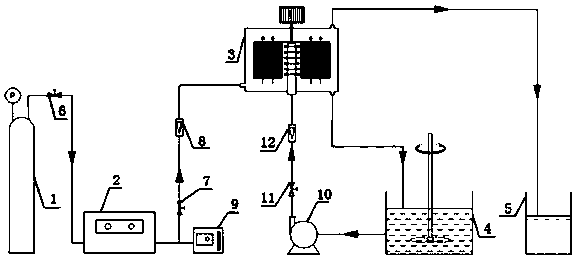
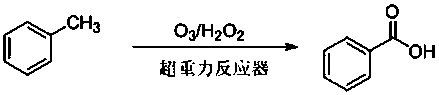Device and method for preparing benzoic acid from toluene
A technology of benzoic acid and toluene, applied in the field of devices for preparing benzoic acid from toluene, achieving broad industrial application prospects and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Weigh 6g of toluene and dissolve it in 500 mL of ethyl acetate and place it in the liquid storage tank. Set the liquid flow meter to 100 L / h, the gas flow meter to 50 L / h, and the gas phase ozone concentration to be 30 mg / L. The speed is 800 rpm. After reacting for 60min, after removing the reaction solvent with a rotary evaporator, through silica gel column chromatography (eluent is V 石油醚 / V 乙酸乙酯 =5:1) to obtain 3.2 g of benzoic acid with a yield of 40.5%.
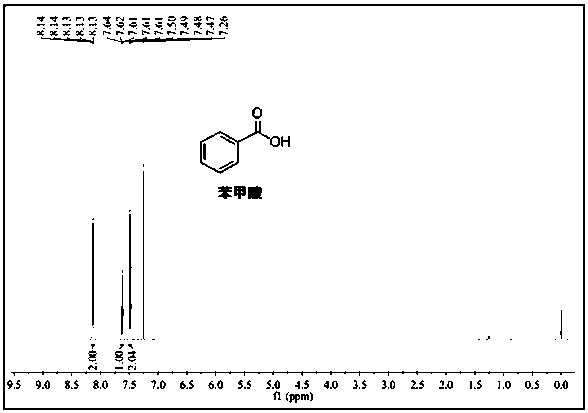
[0026] 1 H NMR (600 MHz, CDCl 3 ) δ 10.02 (s, 1H), 7.88 (d, J = 12 Hz, 2H), 7.63 (t, J = 6 Hz, 1H), 7.53 (t, J = 6 Hz, 2H).
Embodiment 2
[0028] Weigh 6 g of toluene dissolved in 500 mL of methanol and put it in the storage tank, add 1.1 g of hydrogen peroxide (30%), set the liquid flow meter to 100 L / h, the gas flow meter to 70 L / h, and the gas phase ozone concentration to 80 mg / L, the rotation speed of the high gravity reactor is 800rpm. Reaction 80min, after removing reaction solvent with rotary evaporator, through silica gel column chromatography (eluent is V 石油醚 / V 乙酸乙酯 =5:1) to obtain 1.79 g of benzoic acid with a yield of 61%.
[0029] 1 H NMR (600 MHz, CDCl 3 ) δ 10.02 (s, 1H), 7.88 (d, J = 12 Hz, 2H), 7.63 (t, J = 6 Hz, 1H), 7.53 (t, J = 6 Hz, 2H).
Embodiment 3
[0031] Weigh 6 g of toluene and dissolve it in 500 mL of ethyl acetate and place it in the storage tank, add 2.2 g of hydrogen peroxide (30%), set the liquid flow meter to 100 L / h, the gas flow meter to 100 L / h, and the gas phase ozone concentration to be 50 mg / L, the rotation speed of the hypergravity reactor is 500 rpm. After reacting for 120 min, remove the reaction solvent with a rotary evaporator, and perform silica gel column chromatography (eluent is V 石油醚 / V 乙酸乙酯 =5:1) to obtain 6.7 g of benzoic acid with a yield of 85%.
[0032] 1 H NMR (600 MHz, CDCl 3 ) δ 10.02 (s, 1H), 7.88 (d, J = 12 Hz, 2H), 7.63(t, J = 6 Hz, 1H), 7.53 (t, J = 6 Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com