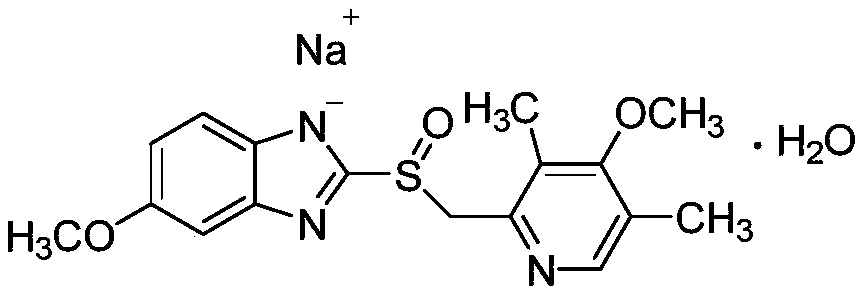
Omeprazole sodium for injection, preparation method and application of omeprazole sodium for prevention of aspiration pneumonitis caused by regurgitation of gastric juice in adaptation disease
A technology for omeprazole sodium and gastric acid reflux, which is applied in the directions of non-active ingredient medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 The medicinal activated carbon used in Chinese medicine is coconut shell medicinal activated carbon, purchased from Zhengzhou Linhai Activated Carbon Co., Ltd., with a mesh size of 8-12 mesh.
[0056] The chitosan used in the examples is of analytically pure grade, with a degree of substitution ≥ 80%, and was purchased from Shanghai Macklin Biochemical Technology Co., Ltd.
[0057] The polyvinylpyrrolidone used in the examples is K30 analytically pure, purchased from Shanghai Abby Chemical Reagent Co., Ltd.
[0058] The polysulfone powder used in the examples is injection molding grade, the place of origin is Solvay, USA, purchased from Shanghai Zhengyong Plastic Chemical Co., Ltd., and the brand name is P-3703.
Embodiment 10
[0059] The hollow fiber ultrafiltration membrane used in Example 10 was purchased from Shenzhen Baideshui Technology Co., Ltd., with a pore size of 5-50 nm.
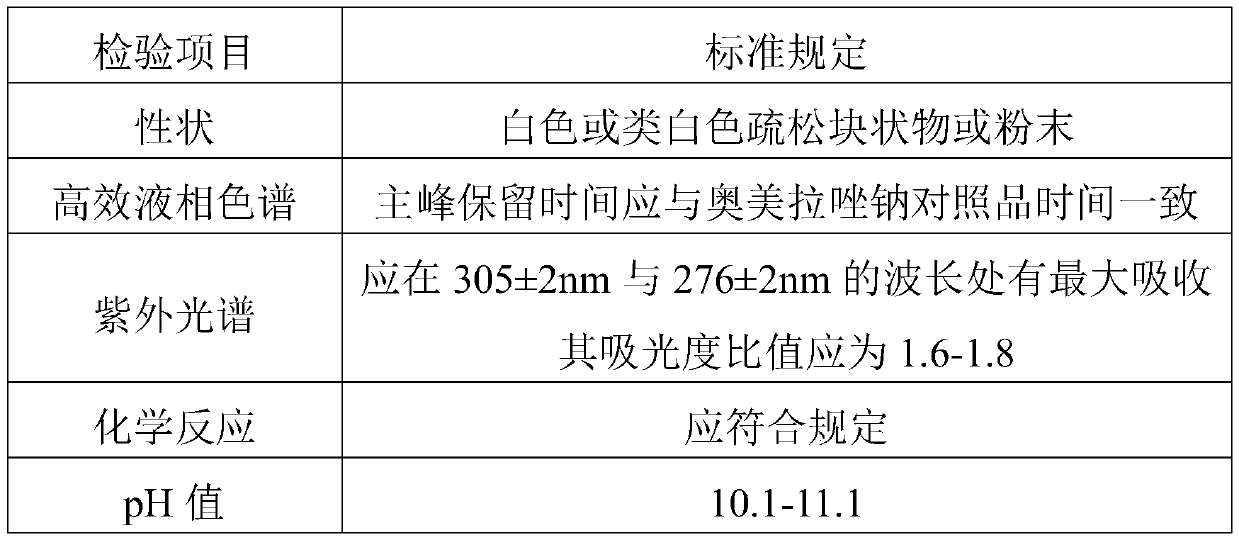
[0060] Carry out long-term stability test to the omeprazole sodium that embodiment 1-9 makes, at temperature 25 ℃ ± 2 ℃, place 6 months under moderate 60% ± 10% condition, every 3 months sampling is successively, respectively at 0 month, 3 months, and 6 months will be tested according to the key items of stability. The inspection basis is the second part of the 2015 edition of the "Chinese Pharmacopoeia" and the State Food and Drug Administration's drug supplement application approval document (approval number: 2012B00303).
[0061] The relevant items for detection of omeprazole sodium for injection are shown in Table 1:
[0062] Table 1 Test table for omeprazole sodium for injection
[0063]
[0064]
[0065] Example 1
[0066] Omeprazole sodium for injection is prepared by adding 21.3 g of omeprazole sodium, 0....
Embodiment 2
[0083] Omeprazole sodium for injection is prepared by adding 21.3 g of omeprazole sodium, 0.75 g of disodium edetate, and adding water for injection to 1000 mL per 1000 dosage unit prescription.
[0084] The preparation method of above-mentioned omeprazole sodium for injection is as follows:
[0085] S1. Add disodium edetate to water for injection with a volume of 80%, stir to dissolve, add omeprazole sodium, continue stirring for 20 minutes, and adjust the pH to 11 with 1.0mol / L sodium hydroxide aqueous solution , adding 0.1% of the mass of the liquid to be adsorbed with medicinal activated carbon for adsorption for 20 minutes, adding the remaining water for injection, and filtering to remove the activated carbon to obtain a semi-finished product;
[0086] S2. Take a sample to detect the content and pH value of the semi-finished product. After passing the inspection, use a 0.22 μm cartridge filter to sterilize and filter, quantitatively fill, and freeze-dry to obtain a freeze...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com