A kind of preparation method of mannose oligosaccharide
A technology of oligomannose and galactomannan, which is applied in the field of preparation of oligomannose, can solve the problems of low selectivity of oligomannose, serious corrosion of inorganic acid equipment, and low yield of oligomannose , to achieve the effect of short reaction time, fast hydrolysis speed and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
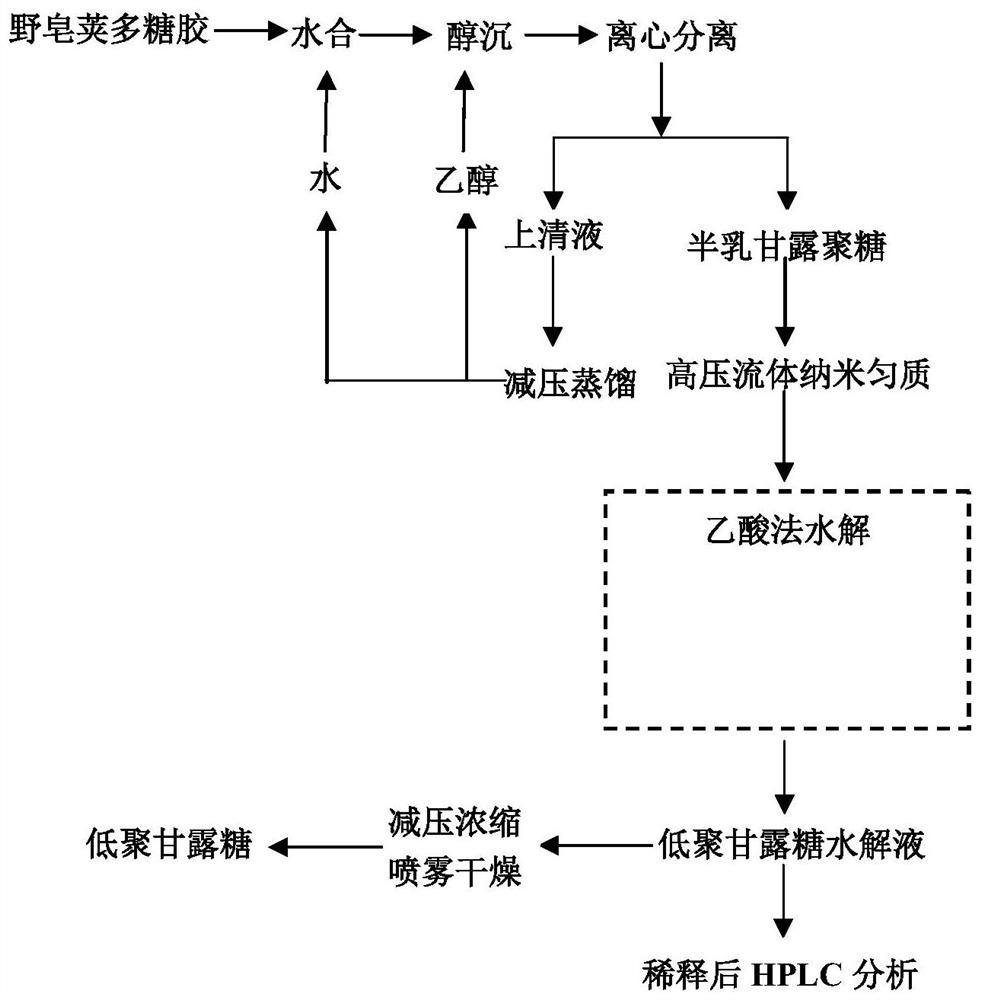
[0033] This example first provides a method for preparing galactomannan, which includes: taking 6 g of industrial acacia polysaccharide gum powder, hydrating it with 300 g at 70°C for 3 hours, centrifuging to remove water-insoluble matter, and then taking the supernatant at a ratio of 1:1 (v / v) Add absolute ethanol under stirring, stand for 2 hours and then centrifuge, and the precipitate is galactomannan. The supernatant can be distilled under reduced pressure to recover ethanol and water.
[0034] This embodiment also provides a method for preparing mannose oligosaccharide, which includes: adding water to the galactomannan prepared in this embodiment to make a solution with a concentration of 10%, and homogenizing it at 200 MPa through a high-pressure fluid nano-homogenizer to homogenize it. The quality-treated galactomannan gum was transferred into the reaction kettle, 20 mL of 5M acetic acid was added, and the reaction was carried out at 130 °C for 2 h to obtain an acetic...
Embodiment 2
[0038] This example provides the preparation method of oligomannose, and the difference from Example 1 is only that the galactomannan treated by high-pressure fluid nano-homogenization is moved into the reaction kettle, 20 mL of 6M acetic acid is added, and the reaction is carried out at 120 ° C for 2 h , to obtain an acetic acid degradation solution; the degraded solution is concentrated in a vacuum to recover acetic acid, and then spray-dried to obtain mannose oligosaccharides.
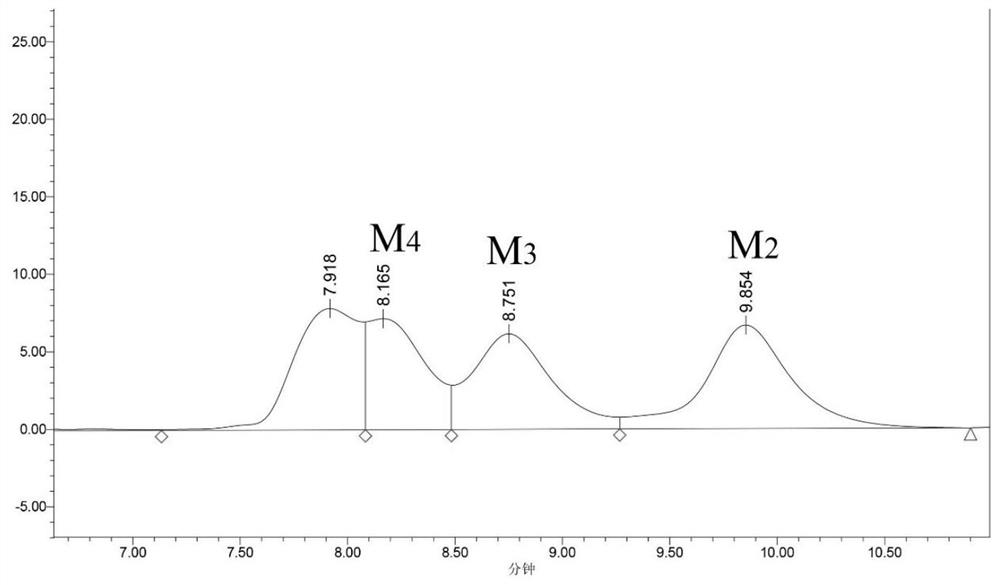
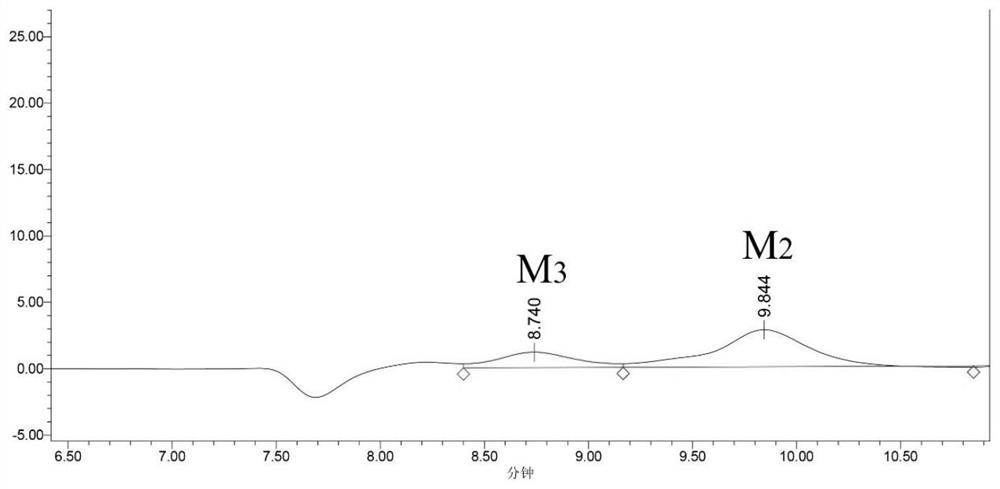
[0039] The acetic acid hydrolyzate obtained in the present embodiment is analyzed by high performance liquid chromatography, wherein the total concentration of oligomannose (mannobiose, mannotriose, mannotetraose) is 32.7g / L, and wherein mannobiose is 10.4g / L. L, mannotriose was 11.7g / L, and mannotetraose was 10.6g / L.
[0040] The total sugar content in the mannose oligosaccharide product prepared in this example is 93.3%.
Embodiment 3
[0042] This example provides the preparation method of oligomannose, the difference from Example 1 is only that the galactomannan treated by high pressure fluid nano-homogenization is moved into the reaction kettle, 20 mL of 6M acetic acid is added, and the reaction is carried out at 130 ° C for 1 h, An acetic acid degradation solution is obtained; the degradation solution is concentrated in a vacuum to recover acetic acid, and is further spray-dried to obtain mannose oligosaccharides.
[0043] The acetic acid hydrolyzate obtained in the present embodiment is analyzed by high performance liquid chromatography, wherein the total concentration of oligomannose (mannobiose, mannotriose, mannotetraose) is 30.5g / L, and wherein mannobiose is 9.6g / L. L, mannotriose was 11.3g / L, and mannotetraose was 9.6g / L.
[0044] The total sugar content in the mannose oligosaccharide product prepared in this example is 93.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com