Acid xylanase mutants with improved enzyme activity and heat resistance, coding gene and application thereof
An acid xylanase and xylanase mutation technology, applied in the field of genetic engineering, can solve the problems of easy inactivation, denaturation inactivation of neutral xylanase, affecting the use effect, etc., and achieve good application potential, enzyme Vitality and thermal stability enhancement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: error-prone PCR constructs the mutant library of acid xylanase XYNTFO
[0043] Referring to the amino acid sequence of acid xylanase XYNTFO (SEQ ID NO: 1), optimize its DNA sequence (SEQ ID NO: 2) according to the codon preference of Pichia pastoris, synthesize the optimized gene, and design EcoR at the 5' end I restriction enzyme cutting site, Not I restriction enzyme cutting site is designed at the 3' end.
[0044] SEQ ID No: 1
[0045]
[0046] SEQ ID No: 2
[0047]
[0048] Use the GeneMorph II Random Mutation PCR Kit and use the xylan XYNTFO gene as a template to perform random mutations. The primer sequences used are as follows:
[0049] XYNTF0F:A GAATTC TTCCCTTCAGAATTG;
[0050] XYNTFOR:A GCGGCCGC TCATGAGACAGTG.
[0051] The amplified random mutation PCR product was double-digested with EcoR I and Not I, purified and recovered, then connected to the pET-21a(+) vector, transformed into Escherichia coli BL21-DE3, and positive clones were...
Embodiment 2
[0059] Example 2: The second round of error-prone PCR constructs a mutant library of xylanase XYNTF01
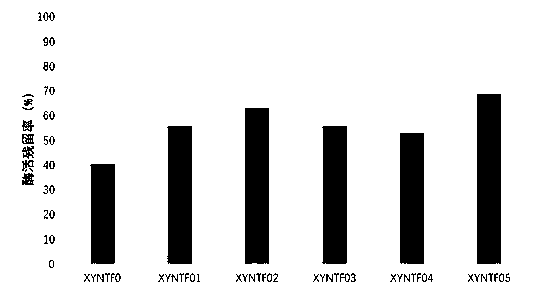
[0060] Using the xylanase gene XYNTF01 screened in Example 1 as a template, the second round of random mutation was carried out. The mutation library construction process, materials, reagents, and operating conditions were the same as in Example 1; mutants were cultivated and screened with XYNTF01 As a control, the remaining activity of the xylanase mutant was detected after heat treatment (80°C water bath for 3 minutes), and the mutant gene with a higher remaining enzyme activity than XYNTF01 was sequenced.
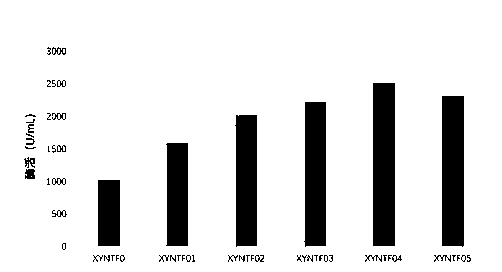
[0061] The following mutants with improved enzyme activity and heat resistance were finally screened:
[0062] The mutation method of XYNTF02 is P84T / S14R, and the amino acid sequence is shown in SEQ ID No: 5;
[0063] The mutation method of XYNTF03 is P84T / L121E, and the amino acid sequence is shown in SEQ ID No: 7;
[0064] The mutation method of XYNTF04 is P84T / S1...
Embodiment 3
[0084] Example 3: Expression verification of xylanase mutants with improved enzyme activity and heat resistance in Pichia pastoris
[0085] The xylanase mutant genes screened above were connected to the pPIC9K plasmid with EcoR I and Not I double restriction sites and transformed into Escherichia coli DH5α, and the expression vector pPIC- XYNm, sequence verified. The expression vectors of each mutant were linearized by digestion with Sal I and then electroporated into Pichia pastoris GS115. The transformants of the expression strains of each mutant were screened on the MD plate and transferred to the YPD plate for activation (each mutant was picked 48 transformants). The activated transformants were placed in 48 deep-well plates (each well containing 1 mL of BMGY medium), cultured with shaking at 30°C for 18 hours, then induced by adding 1% methanol, and continued shaking culture, after which 1% methanol of the culture volume was added every 24 hours. After inducing expressi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com