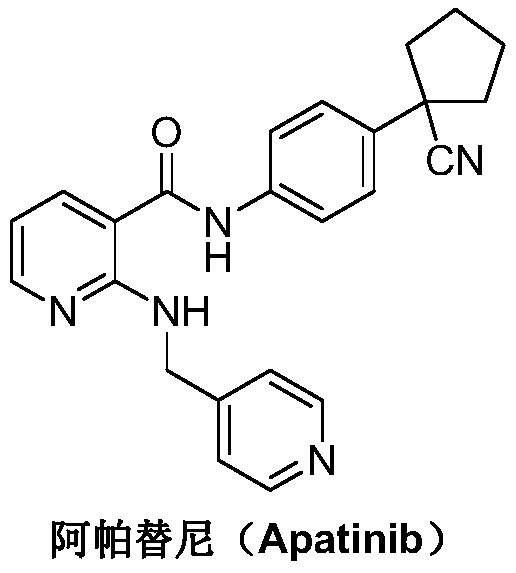
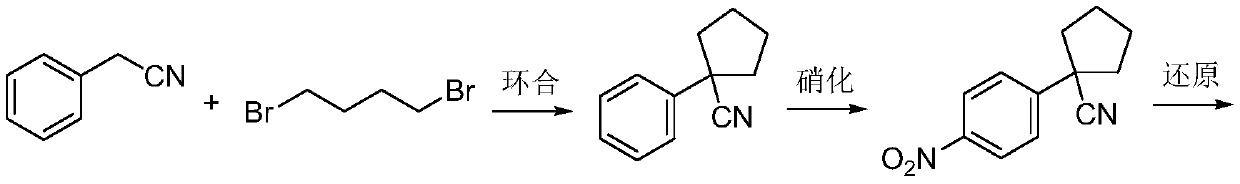
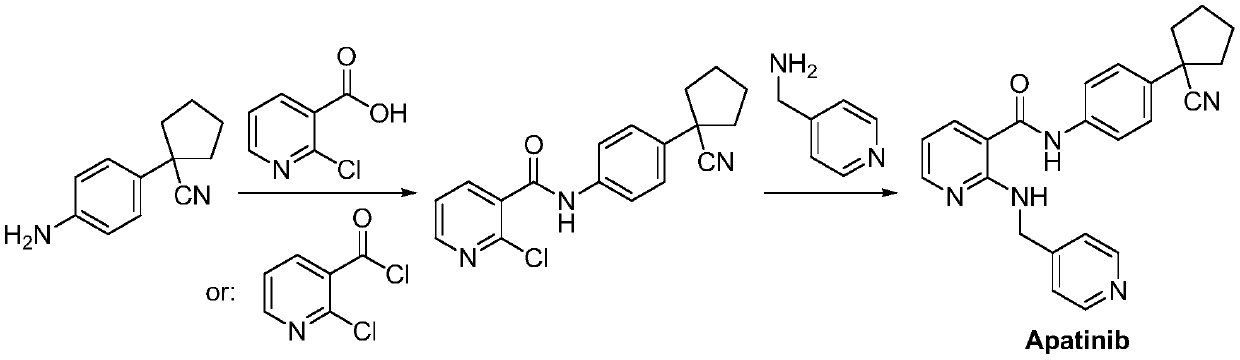
A kind of Apatinib intermediate and preparation method thereof
A technology of apatinib and intermediates, which is applied in the field of medicinal chemical synthesis, can solve the problems of difficult to eliminate impurities, difficult separation and purification, and increased cost, and achieves the effects of less impurities, simplified process method, and optimized reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (A) Preparation of ethyl 4-[(2-chloropyridin-3-yl)carbonylamino]phenylacetate:
[0047] Ethyl 4-aminophenylacetate (55.0g, 0.31mol) was dissolved in N,N-dimethylformamide (1500mL), N,N-diethylaniline (68.7g, 0.46mol) was added, stirred and ice bathed Cool to 5-10°C, add dropwise a solution of 2-chloronicotinoyl chloride (59.4g, 0.34mol) in N,N-dimethylformamide (60mL), after dropping, the reaction mixture rises to 40°C for 10h until the reaction is complete , lowered to room temperature, 1N hydrochloric acid was added dropwise to adjust to pH = 7, the organic solvent was removed by rotary evaporation under reduced pressure, ethyl acetate and water were added for extraction, the organic phase was separated, washed with water and saturated brine successively, dried over anhydrous sodium sulfate, and reduced Concentrated to dryness by rotary evaporation, the resulting crude product was recrystallized with isopropanol and dried to give ethyl 4-[(2-chloropyridin-3-yl)carbony...
Embodiment 2
[0053] (A) Preparation of methyl 4-[(2-chloropyridin-3-yl)carbonylamino]phenylacetate:
[0054] Methyl 4-aminophenylacetate (100.0g, 0.61mol) was dissolved in 1,4-dioxane (2500mL), sodium carbonate (128.3g, 1.21mol) was added, stirred and cooled in an ice bath to 5-10°C, Add dropwise a solution of 2-chloronicotinoyl chloride (159.8g, 0.91mol) in 1,4-dioxane (100mL). After dropping, the reaction mixture rises to 60°C and reacts for 6h until the reaction is complete. After cooling down to room temperature, add 1N Hydrochloric acid was adjusted to pH = 7, the organic solvent was removed by rotary evaporation under reduced pressure, ethyl acetate and water were added for extraction, the organic phase was separated, washed with water and saturated brine successively, dried over anhydrous sodium sulfate, concentrated to dryness by rotary evaporation under reduced pressure, the obtained The crude product was recrystallized with isopropanol and dried to obtain methyl 4-[(2-chloropyrid...
Embodiment 3
[0060] (A) Preparation of methyl 4-[(2-chloropyridin-3-yl)carbonylamino]phenylacetate:
[0061] Methyl 4-aminophenylacetate (153.0g, 0.93ol) was dissolved in chloroform (3500mL), added pyridine (131.9g, 1.67mol), stirred and cooled to 5-10°C in an ice bath, and 2-chloronicotinoyl chloride ( 211.9g, 1.20mol) of chloroform (250mL) solution, after dropping, the reaction mixture was incubated at 20°C for 16h until the reaction was complete, then cooled to room temperature, 1N hydrochloric acid was added dropwise to adjust the pH to 7, the organic solvent was removed by rotary evaporation under reduced pressure, and the Extracted with ethyl acetate and water, separated the organic phase, washed with water and saturated brine successively, dried over anhydrous sodium sulfate, concentrated to dryness by rotary evaporation under reduced pressure, the obtained crude product was recrystallized with isopropanol, dried to give 4-[(2 -Chloropyridin-3-yl)carbonylamino]phenylacetic acid meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com