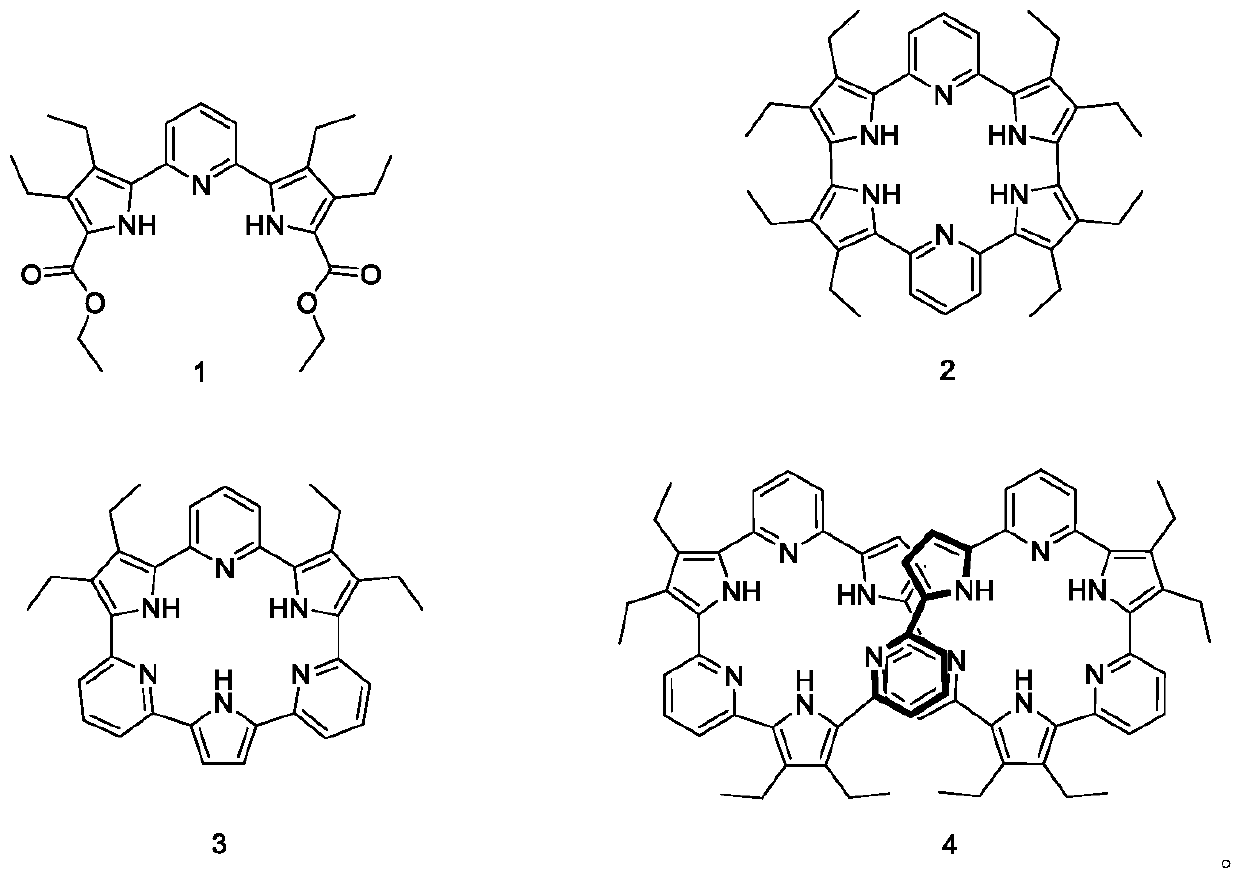
Post-treatment purification method of pyrrole-pyridine-pyrrole compound
A purification method and compound technology, applied in the direction of organic chemistry, can solve the problems of time-consuming, consumption, multi-solvent, etc., and achieve the effect of shortening the reaction time, high product purity, and improving the purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
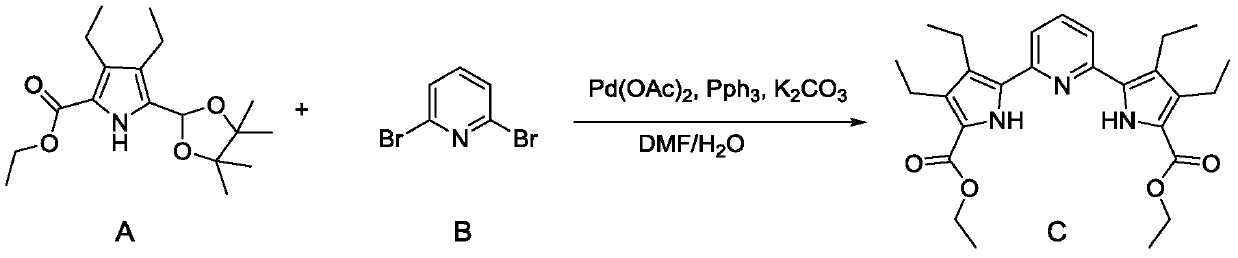
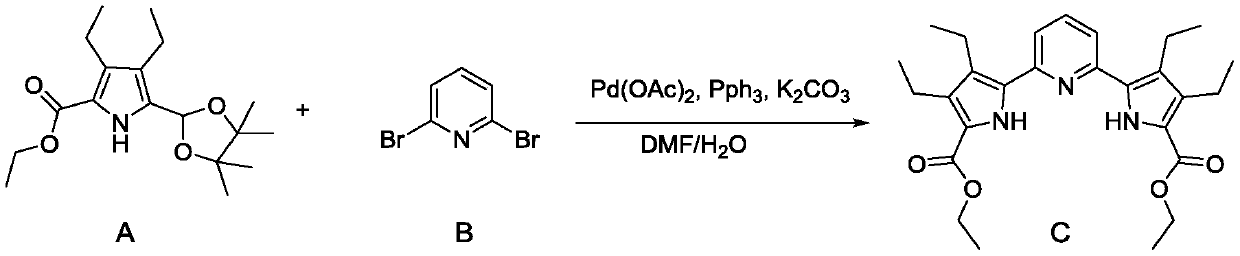
[0026] This example expands the synthesis of a pyrrole-pyridine-pyrrole compound as an example of a porphyrin intermediate. The synthetic reaction equation is as follows:
[0027]
[0028] Including the following steps:
[0029] (1) Add compound B (0.3070g), palladium acetate (0.0291g), triphenylphosphine (0.0681g) and potassium carbonate (0.5916g) in a round bottom flask of appropriate size;
[0030] (2) remove the air in the bottle under reduced pressure, blow nitrogen, and repeat three times;
[0031] (3) Add N,N-dimethylformamide (20mL) and water (10mL) under nitrogen protection;
[0032] (4) The system was stirred and reacted at 85°C for about 1 hour;
[0033] (5) A solution of N,N-dimethylformamide (10 mL) dissolved in Compound A (1.0 g) was slowly added dropwise to the system;
[0034] (6) After the dropwise addition, the system was stirred and reacted at 85° C. for 24 hours under the protection of nitrogen;
[0035] (7) reaction finishes, and decompression remov...
Embodiment 2
[0041] This example expands the synthesis of a pyrrole-pyridine-pyrrole compound as an example of a porphyrin intermediate. The synthetic reaction equation is as follows:
[0042]
[0043] Including the following steps:
[0044] (1) Add compound B (0.614g), palladium acetate (0.0582g), triphenylphosphine (0.1362g) and potassium carbonate (1.1832g) in a round bottom flask of appropriate size;
[0045] (2) remove the air in the bottle under reduced pressure, blow nitrogen, and repeat three times;
[0046] (3) Add N,N-dimethylformamide (40mL) and water (20mL) under nitrogen protection;
[0047] (4) The system was stirred and reacted at 85°C for about 1 hour;
[0048] (5) A solution of N,N-dimethylformamide (20 mL) dissolved in Compound A (2.0 g) was slowly added dropwise to the system;
[0049] (6) After the dropwise addition, the system was stirred and reacted at 85° C. for 24 hours under the protection of nitrogen;
[0050] (7) reaction finishes, and decompression remove...
Embodiment 3
[0056] This example expands the synthesis of a pyrrole-pyridine-pyrrole compound as an example of a porphyrin intermediate. The synthetic reaction equation is as follows:
[0057]
[0058] Including the following steps:
[0059] (1) Add compound B (1.5350g), palladium acetate (0.1455g), triphenylphosphine (0.3405g) and potassium carbonate (2.9580g) in a round bottom flask of appropriate size;
[0060] (2) remove the air in the bottle under reduced pressure, blow nitrogen, and repeat three times;
[0061] (3) Add N,N-dimethylformamide (100mL) and water (50mL) under nitrogen protection;
[0062] (4) The system was stirred and reacted at 85°C for about 1 hour;
[0063] (5) A solution of N,N-dimethylformamide (50 mL) dissolved in compound A (5.0 g) was slowly added dropwise to the system;
[0064] (6) After the dropwise addition, the system was stirred and reacted at 85° C. for 24 hours under the protection of nitrogen;
[0065] (7) reaction finishes, and decompression remove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com