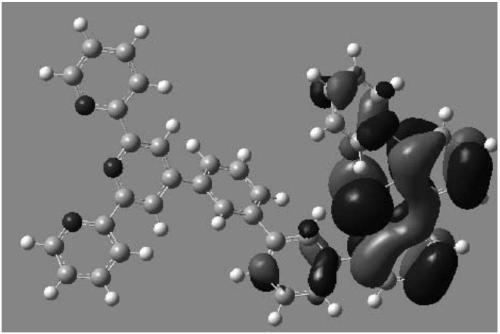
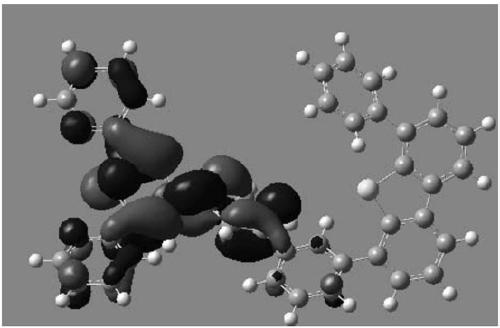
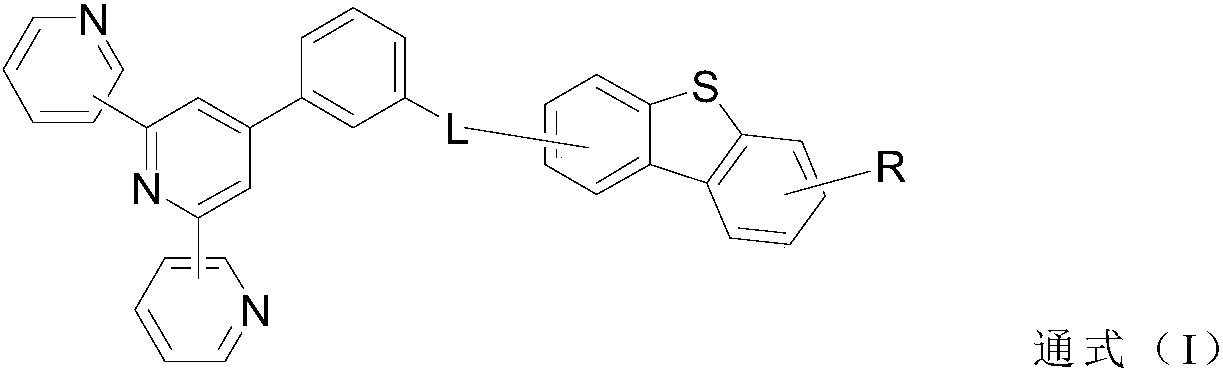
Compound and OLED (organic light emitting device) using same
A compound and selected technology, applied in the direction of electro-solid devices, electrical components, light-emitting materials, etc., can solve the problems of low device efficiency, undeveloped organic electroluminescent device compounds, and poor carrier balance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0079] compound synthesis
[0080] The compounds of the present invention can be obtained by the following two synthetic routes.
[0081]
[0082]
[0083]
[0084]
[0085] By substituting different ones of the aforementioned group A (which is sometimes referred to in the art as boronic acid), different target compounds can be obtained. It should be noted that Suzuki coupling is used in the above synthesis method, but is not limited to this coupling method. Those skilled in the art can choose other methods, such as Stille coupling method, Grignard reagent method, Kumada-Tamao and other known methods.
[0086] Specifically, synthesis methods of representative compounds A2, A5, A6, A11 and A29 of the present invention are shown below.
Synthetic example 1
[0087]
[0088]
[0089] Add 39.3g (173mmol) of 4-bromodibenzothiophene, 30g (157mmol) of phenylboronic acid, 0.9g (0.785mmol, 0.5%) of tetrakis(triphenylphosphine palladium), 1500mL of toluene, 1000mL of ethanol, carbonic acid 43.3 g (314 mmol) of potassium and 1000 mL of water were reacted at 80° C. for 3.5 hours. After the reaction is complete, stop the reaction. After cooling to room temperature and filtering, the obtained solid was purified by recrystallization in toluene to obtain a white powder.
[0090] in N 2 Added 36g (190mmol) of 4-phenyl-dibenzothiophene and 2500mL THF under protection, cooled the temperature to -40°C with liquid nitrogen in an ice ethanol bath, clarified the reaction solution, and began to dropwise add 95mL (228mmol) of n-butyllithium ). No obvious temperature rise was observed during the dropwise addition, the color of the reaction solution became darker to reddish brown, and a small amount of solid precipitated out. When 1 / 2 of the n-bu...
Synthetic example 2
[0095]
[0096]
[0097] Add 39.3 g (173 mmol) of 3-bromodibenzothiophene, 30 g (157 mmol) of 4-pyridineboronic acid, 0.9 g (0.785 mmol, 0.5%) of tetrakis(triphenylphosphine palladium), 1500 mL of toluene, and 1000 mL of ethanol into the reaction flask , 43.3 g (314 mmol) of potassium carbonate, and 1000 mL of water were reacted at 80° C. for 3.5 hours. After the reaction is complete, stop the reaction. After cooling to room temperature and filtering, the obtained solid was purified by recrystallization in toluene to obtain a white powder.
[0098] in N 2 Add 36g (190mmol) 3-(4-pyridine)-dibenzothiophene and 2500mL THF under protection, cool down to -40°C with liquid nitrogen in an ice ethanol bath, clarify the reaction solution, and start adding 95mL of n-butyllithium dropwise (228 mmol). No obvious temperature rise was observed during the dropwise addition, the color of the reaction solution became darker to reddish brown, and a small amount of solid precipitated out...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com