Long-acting low-toxicity recombinant anti-VEGF humanized monoclonal antibody and production method thereof
A technology of humanization and antibodies, which is applied in chemical instruments and methods, biochemical equipment and methods, antibodies, etc., can solve the problems of patient burden, short vitreous half-life, etc., to prolong the treatment cycle and improve the physiological and psychological conditions of patients , highly stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
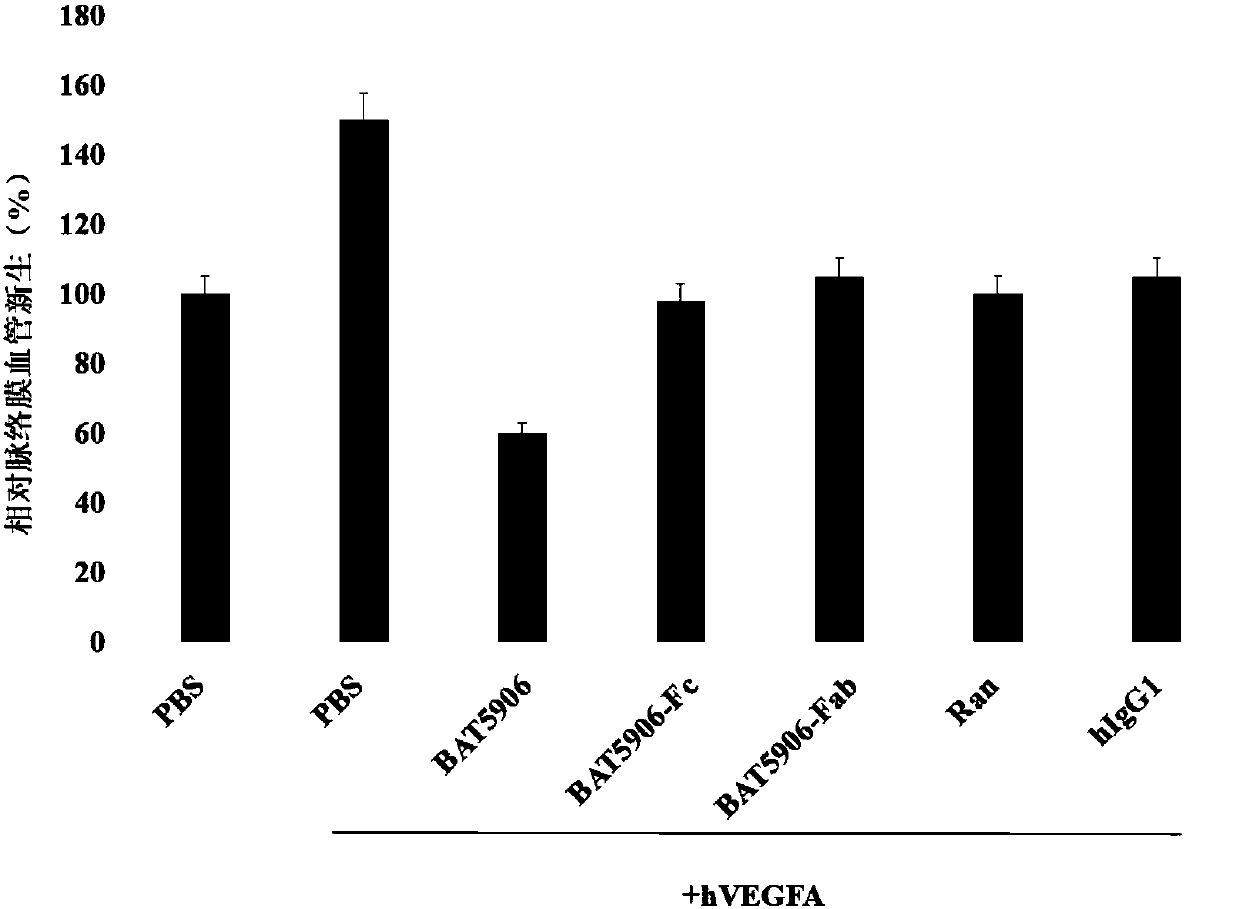
[0091] Example 1 Research on the Inhibitory Mechanism of Recombinant Humanized Anti-VEGF Antibody on Neovascularization
[0092] The heavy chain and light chain amino acid sequences of the recombinant anti-VEGF humanized monoclonal antibody (antibody BAT5906) are shown in SEQ ID NO: 1 and SEQ ID NO: 2, respectively.
[0093] Laser photocoagulation was performed on the eyes of mice to destroy the choroidal structure and form an angiogenesis model. After injecting human VEGFA antigen into this model, the choroidal angiogenesis will be enhanced; then the recombinant humanized anti-VEGF monoclonal antibody BAT5906, Ranibizumab (Ran), Fab domain of BAT5906 antibody (BAT5906-Fab), Fc Domain (BAT5906-Fc) and human IgG1 (hIgG1), compared with the control group (PBS), choroidal angiogenesis were inhibited, presumably there are two different mechanisms of angiogenesis inhibition. Among them, BAT5906-Fc and human IgG1 showed non-target-dependent inhibition, Ranibizumab and BAT5906-Fab sho...
Embodiment 2
[0095] Embodiment 2 Recombinant plasmid and stable cell line construction of recombinant anti-VEGF monoclonal antibody (antibody BAT5906)
[0096] The recombinant expression plasmid pBAT5906 was constructed according to the amino acid sequence, which contains GS cDNA elements for the synthesis of glutamine synthetase gene, which is used as an amplification and screening target for stable cell lines, so that a certain amount of L-methionine subunit can be added to the culture medium. Sulfone (methionine sulphoximine, MSX) was used to screen stable cell lines. The constructed recombinant expression plasmid pBAT5906 was verified by restriction endonuclease Pvu I / Not I digestion, which was consistent with the expected design results, proving that the pBAT5906 recombinant expression vector was successfully constructed.
[0097] The host cell line used for antibody expression is a derivative cell line of CHO-K1 cells, grown in suspension in CD-CHO medium. The construction process o...
Embodiment 3
[0098] Embodiment 3 Expression and purification of monoclonal antibody
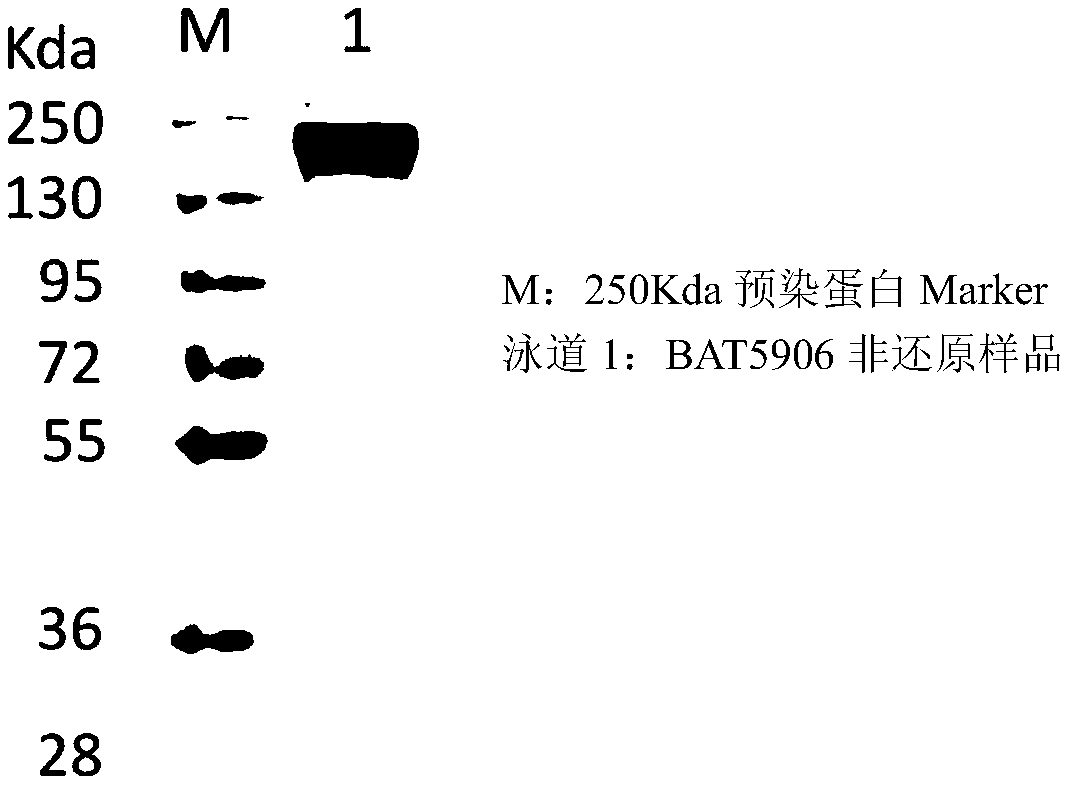
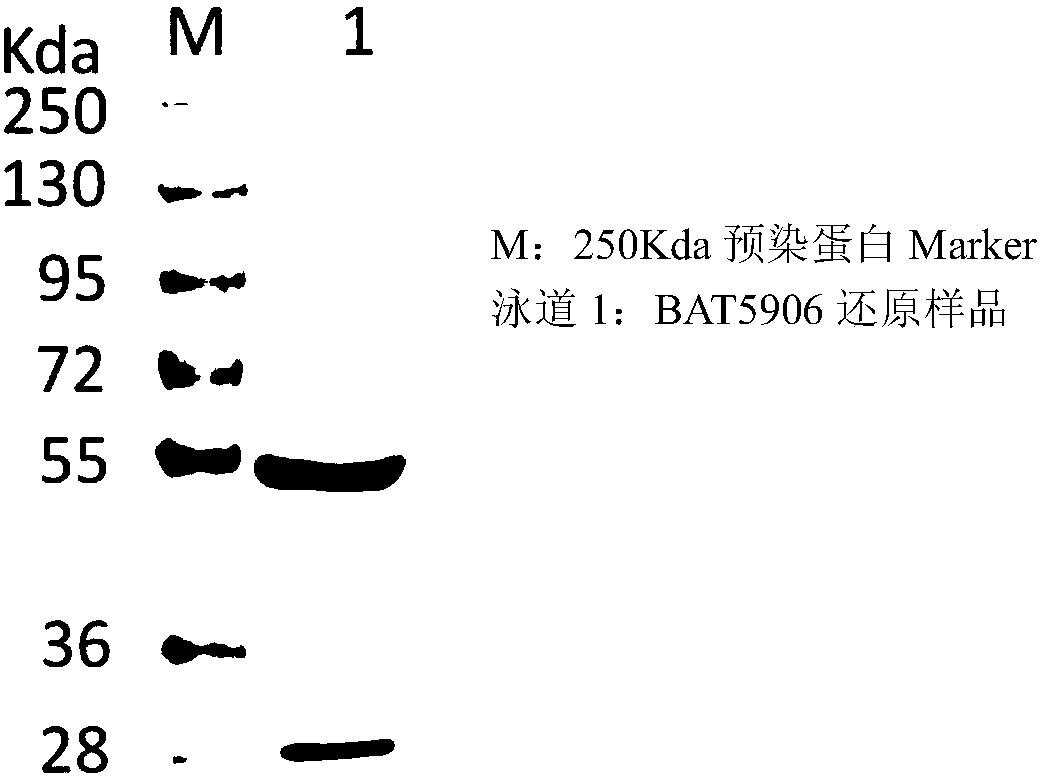
[0099] The expression and purification process of the monoclonal antibody is as follows: After the cells are cultured on a large scale for 2 weeks, the cells and the medium are separated by low-speed centrifugation, and the harvested supernatant is further centrifuged at high speed to obtain a clarified feed solution. The recombinant antibody was purified by two-step affinity chromatography (Protein A) and ion exchange. The media used in the purification were MabSelect SuRe LX produced by GE, Giga Cap Q-650M produced by TOSOH and POROS XS produced by ABI. . The purified antibody was verified by SDS-PAGE to verify its correct size ( figure 2 and image 3 ), the results showed that the BAT5906 band was of the correct size under both reduced and non-reduced conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com