Method for preparing poly-vinyl ether compound through organic-base catalysis
A catalytic preparation and organic base technology, applied in the field of organic synthesis, can solve problems such as slow progress, and achieve the effects of convenient storage and use, good thermal stability, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
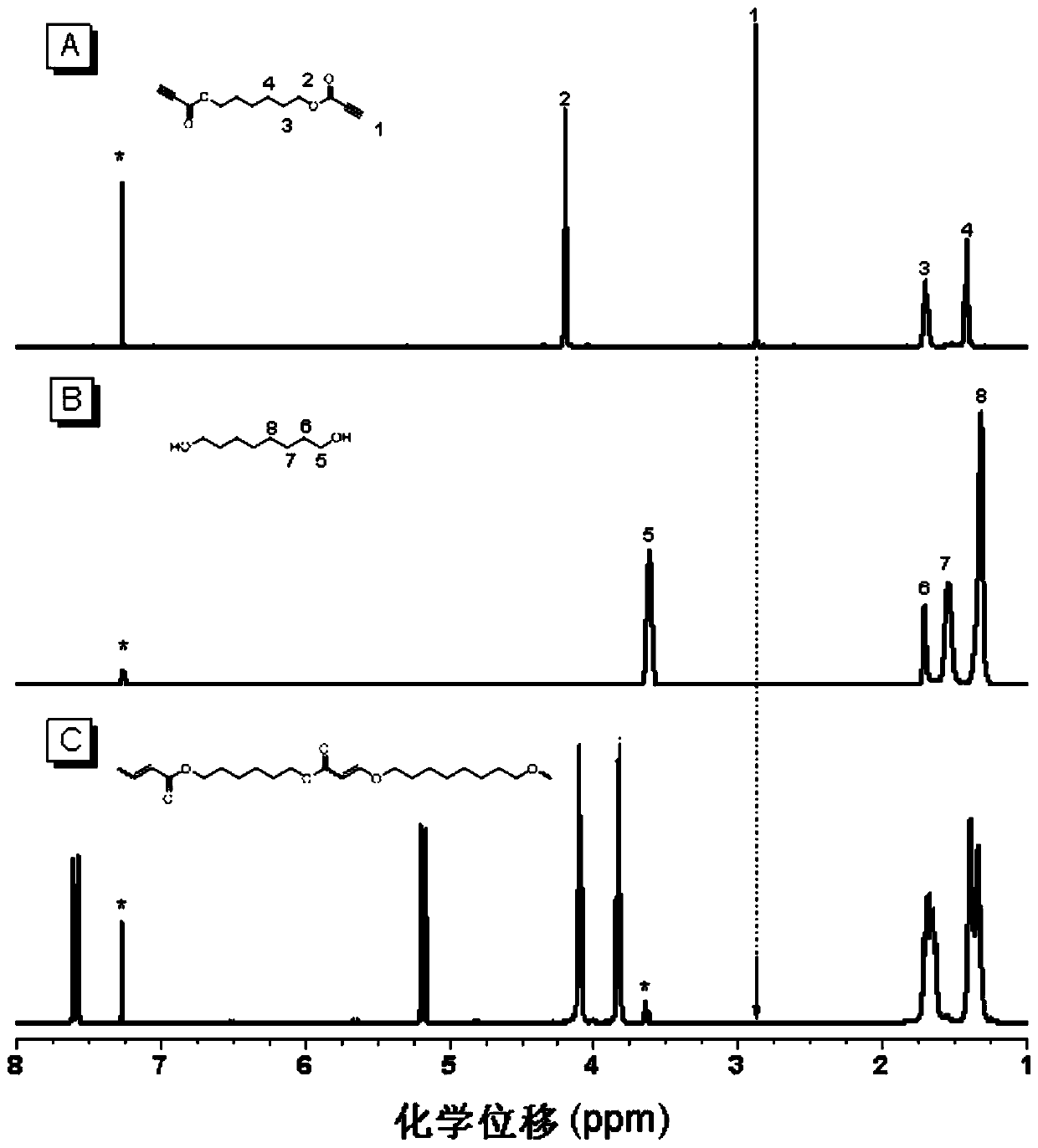
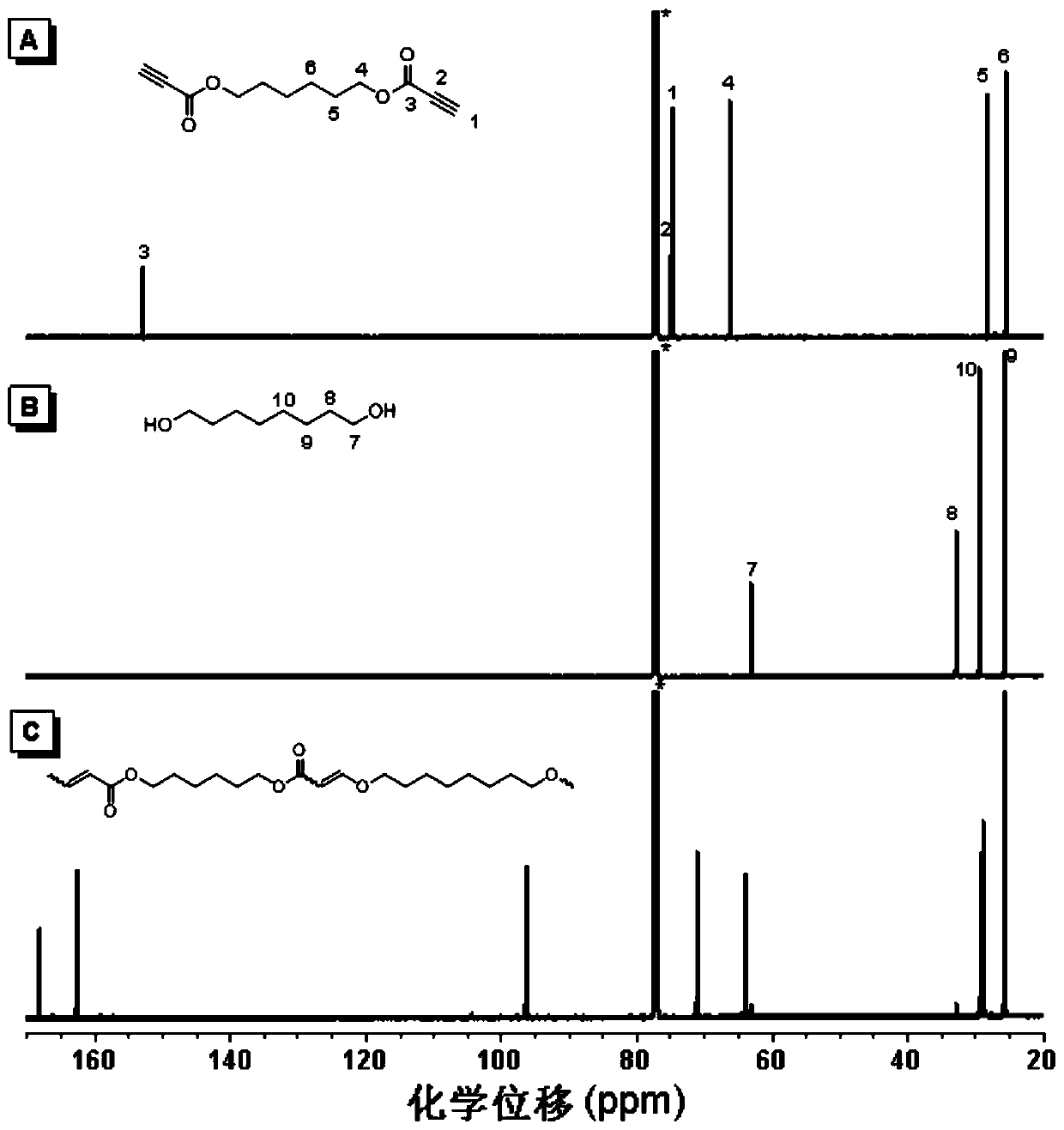
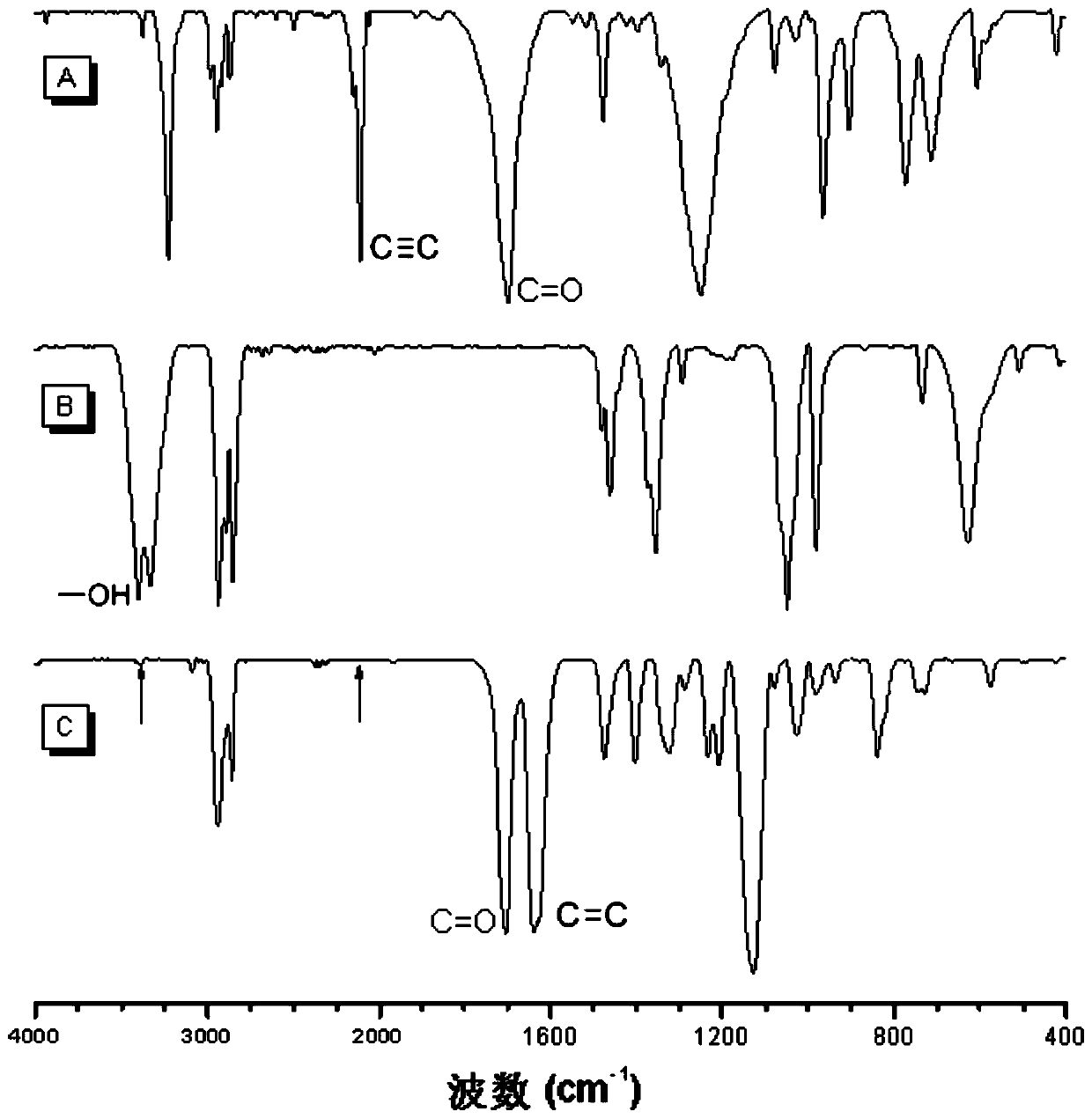
[0035] A polyalkenyl ether compound P1 in this embodiment is prepared by click alkoxylation polymerization of a di-alkyne compound and a di-hydroxy compound, and the reaction equation is as formula (1):
[0036]
[0037] Wherein, the synthesis method of monomer M1 can be synthesized according to the synthesis method of the applicant in the published literature (Polym.Chem., 2012, 3, 1075-1083); M2 is 1,8-octanediol, which can be purchased from the market In this example, it was purchased from Aladdin Company; the catalyst DABCO can be purchased from the market, and in this example and the examples described below, it was purchased from TCI (Shanghai) Chemical Industry Development Co., Ltd.
[0038] The preparation steps of described polyalkene ether compounds are as follows:
[0039] Add 44.4 mg (0.2 mmol) of monomer M1, 29.2 mg (0.2 mmol) of monomer M2 and 0.4 mL of tetrahydrofuran into a 10 mL polymerization tube. After the monomers are completely dissolved, the temperatu...
Embodiment 2
[0042] A polyalkenyl ether compound P2 in this example is prepared by click alkoxylation polymerization of a di-alkyne compound and a di-hydroxy compound, and the reaction formula is as follows (2):
[0043]
[0044] Wherein, the synthesis method of the monomer M1 is the same as that of Example 1; M3 is 1,12-dodecanediol, which can be purchased from the market, and in this example is purchased from Aladdin Company.
[0045] The preparation steps of described polyalkene ether compounds are as follows:
[0046] Add 44.4 mg (0.2 mmol) of monomer M1, 40.5 mg (0.2 mmol) of monomer M3 and 0.4 mL of tetrahydrofuran into a 10 mL polymerization tube. After the monomers are completely dissolved, the temperature is raised to 25°C. 2.24mg (0.02mmol) DABCO was dissolved in 0.1mL tetrahydrofuran. After the system was kept at constant temperature, the DABCO solution was added to the above monomer solution and reacted for 1 hour. After the reaction was finished, the reaction solution was ...
Embodiment 3
[0049] A polyalkenyl ether compound P3 in this embodiment is prepared by click alkoxylation polymerization of a di-alkyne compound and a di-hydroxy compound, and the reaction formula is as formula (3):
[0050]
[0051] Wherein, the synthesis method of monomer M1 is the same as in Example 1; M4 is dibromoneopentyl glycol, which can be purchased from the market, and in this example, it is purchased from Aladdin Company.
[0052] The preparation steps of described polyalkene ether compounds are as follows:
[0053] Add 44.4 mg (0.2 mmol) of monomer M1, 52.4 mg (0.2 mmol) of monomer M4 and 0.4 mL of tetrahydrofuran into a 10 mL polymerization tube. After the monomers are completely dissolved, the temperature is raised to 25°C. 2.24mg (0.02mmol) DABCO was dissolved in 0.1mL tetrahydrofuran. After the system was kept at constant temperature, the DABCO solution was added to the above monomer solution and reacted for 1 hour. After the reaction, the reaction solution was diluted w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com