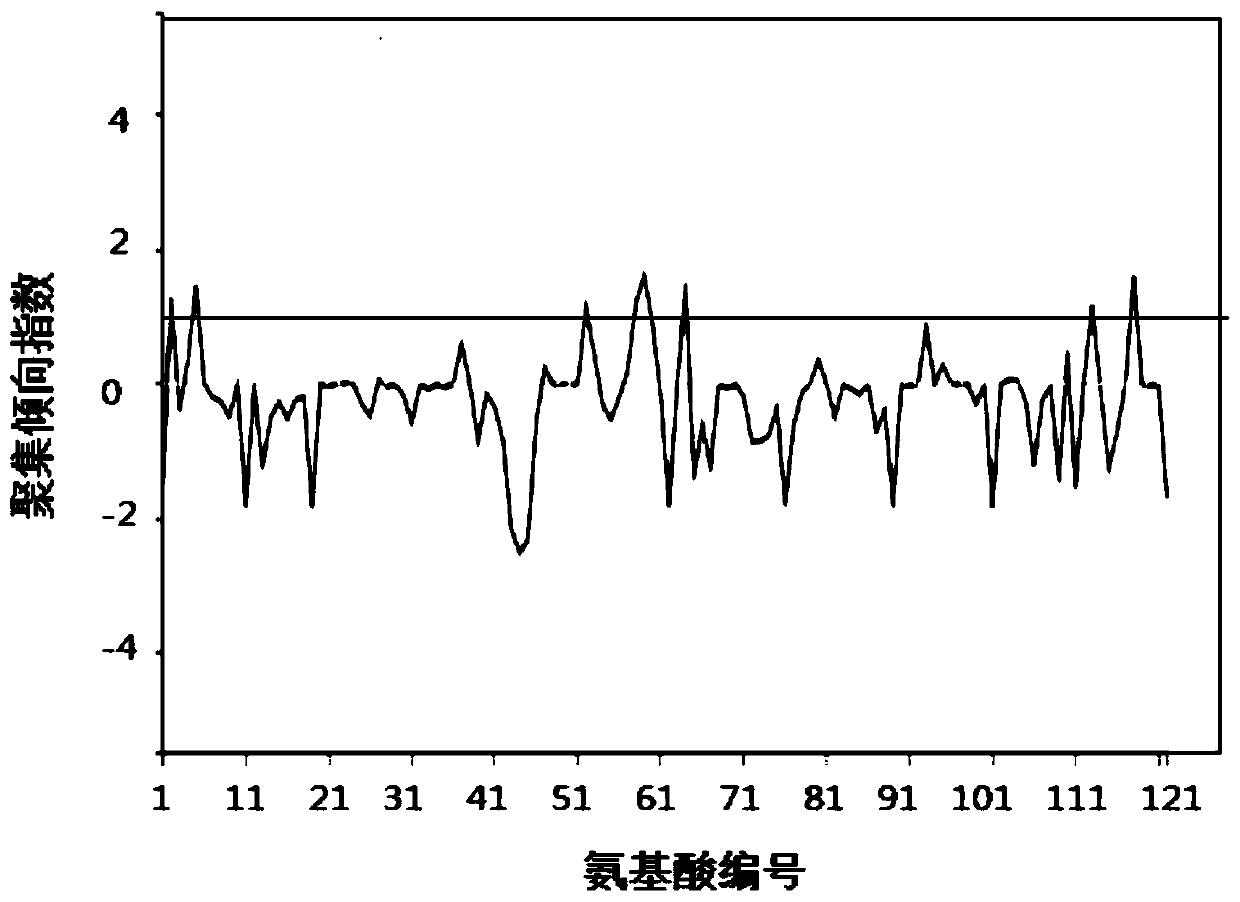
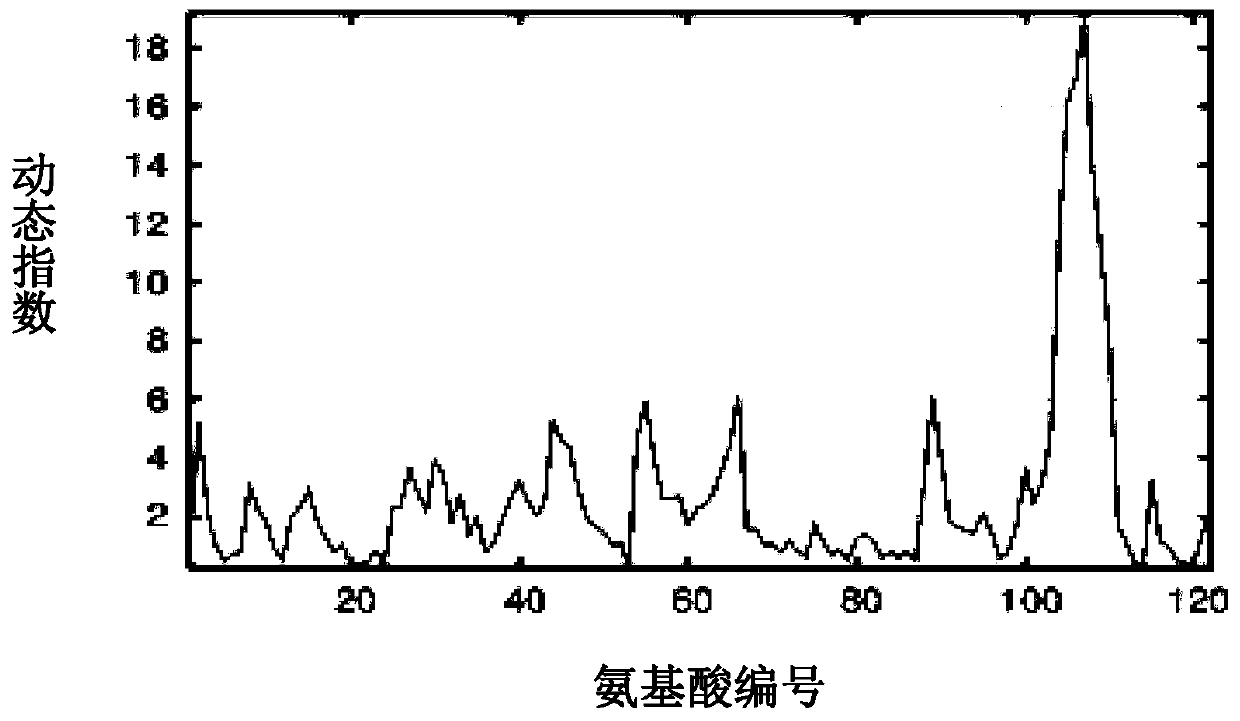
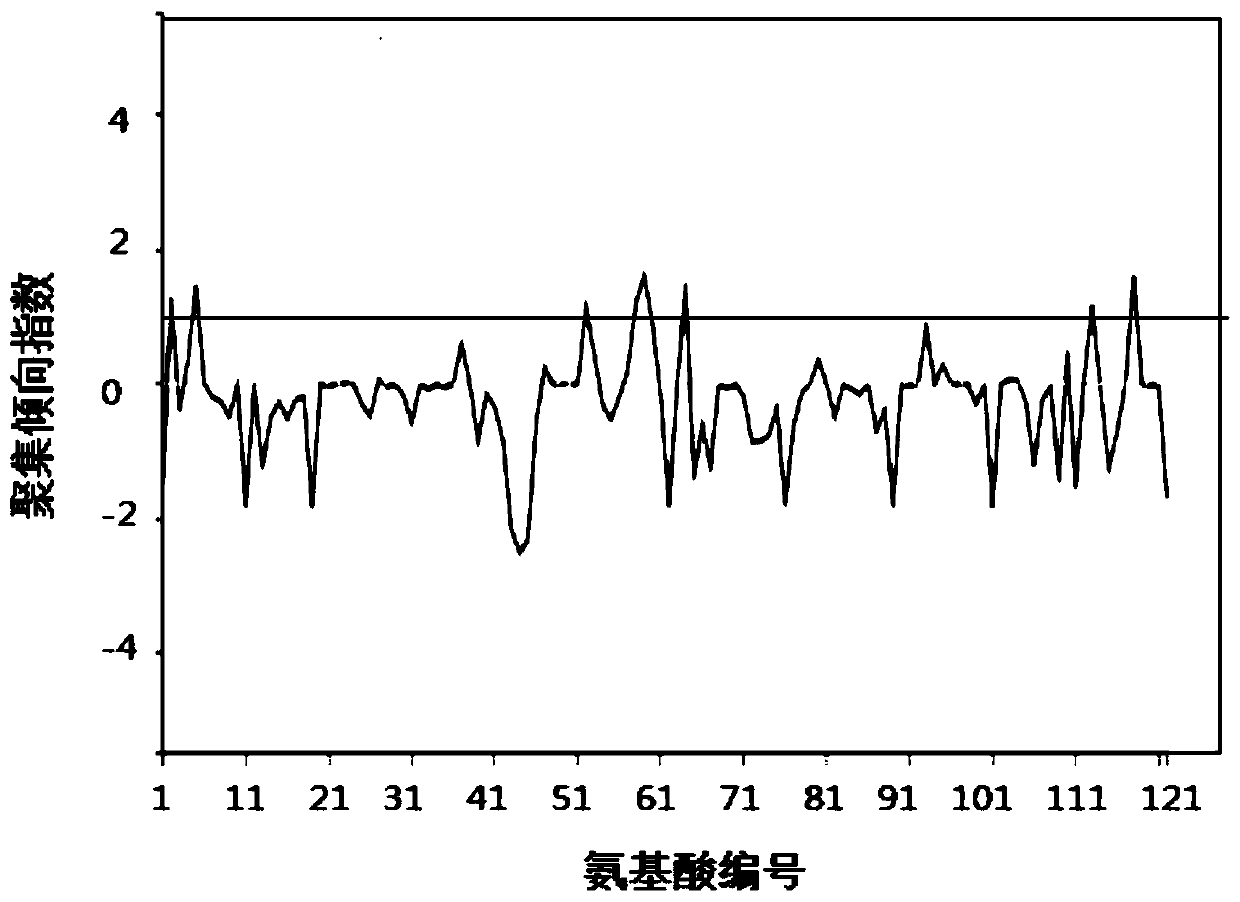
Protein drug auxiliary screening method
A screening method and protein technology, applied in the field of protein drugs, can solve the problems of high cost of rework, screening out, and platform methods are limited to regular antibodies, etc., and achieve the effect of stable and reliable screening results, short time-consuming, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] The candidate excipients of the therapeutic protein DR5 antibody are screened according to the protein drug excipient screening method of the present invention, and the obtained dissociation energy values are shown in Table 1 and Table 2 below.
[0081] The candidate excipients include proline, alanine, arginine, glutamic acid, glycine, histidine, mannitol, sucrose, trehalose, sorbitol, citric acid, acetic acid, succinic acid, among which, lemon Acid, acetic acid, succinic acid, and histidine are pH buffering agents, and proline, alanine, arginine, glutamic acid, glycine, mannitol, sucrose, trehalose, and sorbitol are non-pH control auxiliary materials.
[0082] Table 1 Sort of pH buffer
[0083] pH buffer Dissociation energy Absolute value of dissociation energy Citric acid-7.57.5 Succinic acid-5.85.8 Histidine-4.84.8 Acetic acid-4.14.1
[0084] The data in Table 1 shows that the pH buffer with the best stabilizing effect on the therapeutic protein DR5 antibody is a...
Embodiment 2
[0093] The candidate excipients of the therapeutic protein-quaternary Fc-fusion drug molecule Anti-DR5 antibody (abbreviated as DR5 antibody) were screened by routine stability experiments. The experimental results obtained are shown in Table 3a to Table 10 below.
[0094] This example 2 used 4 kinds of protein stabilization systems added with 4 kinds of pH buffering agents respectively, the 4 kinds of protein stabilization systems including citric acid system, acetic acid system, succinic acid system and histidine system, the citric acid system The auxiliary material components and their concentrations and the pH value of the citric acid system are shown in Table 3a. The auxiliary material components and their concentrations of the acetic acid system and the pH value of the acetic acid system are shown in Table 3b. The auxiliary material components of the succinic acid system and The concentration and the pH value of the succinic acid system are shown in Table 3c, and the auxilia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com