Method for preparing iron-sulfide-supported alpha-crystal-form iron oxyhydroxide catalyst
A technology of iron oxyhydroxide and catalyst, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., to achieve the effect of improving efficiency, high catalytic ability, and improving catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Firstly, an iron sulfide-supported crystalline iron oxyhydroxide catalyst was prepared.
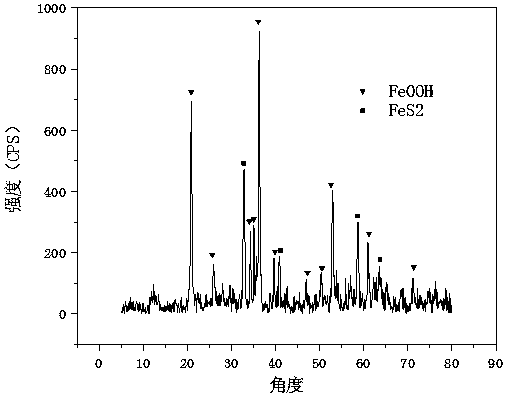
[0043] Such as figure 1 As shown, a one-step hydrothermal method was used to synthesize the obtained FeS 2 It is nanoscale, and α-FeOOH is nanoscale. The specific synthesis method is as follows:
[0044] (1) Accurately weigh 0.84g ferrous sulfate heptahydrate (FeSO 4 ·7H 2 O), it was completely dissolved in 35ml deionized water by stirring; the molar concentration was 0.15mol / L.
[0045] (2) Accurately weigh 2.92g of anhydrous sodium sulfite (Na 2 SO 3 ), which was completely dissolved in 25ml deionized water by stirring; the molar concentration was 1.2mol / L
[0046] (3) Slowly add the dissolved sodium sulfite solution dropwise into the ferrous sulfate solution under stirring, and fully stir for one hour at a speed of 300±30r / min;
[0047] (4) After the stirring is completed, slowly pour the slurry into a stainless steel high-pressure reactor lined with polytetrafluoroethyle...
Embodiment 2
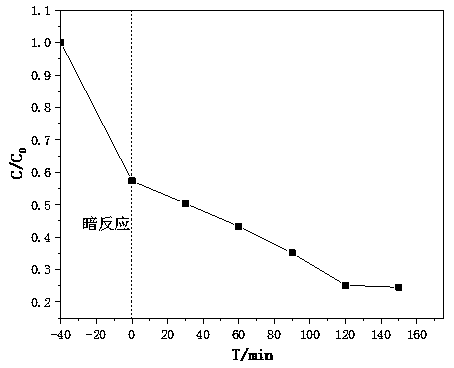
[0063] Degradation of heavy metal Cr in water over iron sulfide-supported crystalline iron oxyhydroxide catalyst 6+ Methods:
[0064] Effectively degrade heavy metal ions in water under acidic conditions, utilize potassium dichromate to prepare 100mg / L chromium ion solution for subsequent use, get 15mL preparation solution, then get 35mL deionized water, prepare 50mL concentration as chromium ion solution of 30mg / L, pass Dilute hydrochloric acid is used to adjust the pH value of the solution between 2-4.
[0065] 1. Add the treated chromium ion solution to the photocatalytic reactor, then add nano-semiconductor photocatalyst to the photocatalytic reactor, and form a mixture in the photocatalytic reactor; stir the solution at a speed of 200±20r / min , to form a uniform slurry, cover the photocatalytic reactor with a dark reaction hood, provide a dark reaction environment, and stir for a period of time under no light conditions to allow the composite photocatalyst to fully absor...
Embodiment 3
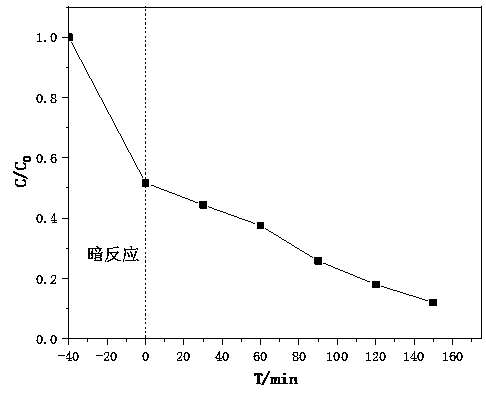
[0073] The method of effectively degrading the macromolecular dye Rhodamine B in water under acidic conditions:
[0074] Specifically: use Rhodamine B to prepare a 100mg / L dye solution for later use, take 15mL of the prepared solution, then take 35mL of deionized water, prepare 50mL of a dye solution with a concentration of 30mg / L, and adjust the pH value of the solution to 2 with dilute hydrochloric acid. Between -4.
[0075] 1. Add the treated dye solution to the photocatalytic reactor, and then add nano-semiconductor photocatalyst to the photocatalytic reactor to form a mixture in the photocatalytic reactor; stir the solution at a speed of 200±20r / min, Make it form a uniform slurry, cover the photocatalytic reactor with a dark reaction hood, provide a dark reaction environment, and stir for a period of time under no light conditions to allow the composite photocatalyst to fully absorb chromium ions in water. After the adsorption is complete, take the upper solution to meas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com