Affinity purification process capable of efficiently improving resolution of polymer separation
A polymer and resolution technology, applied in the field of affinity purification technology, can solve problems such as protein instability, lower purification pressure, low pH elution conditions, etc., and achieve a wide range of applications and remarkable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
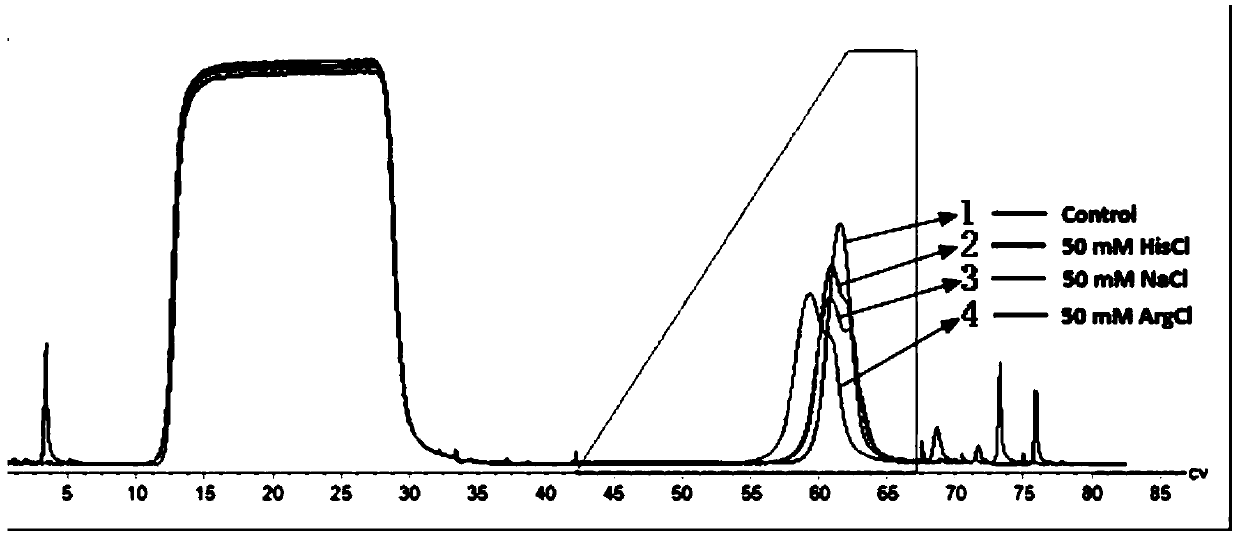
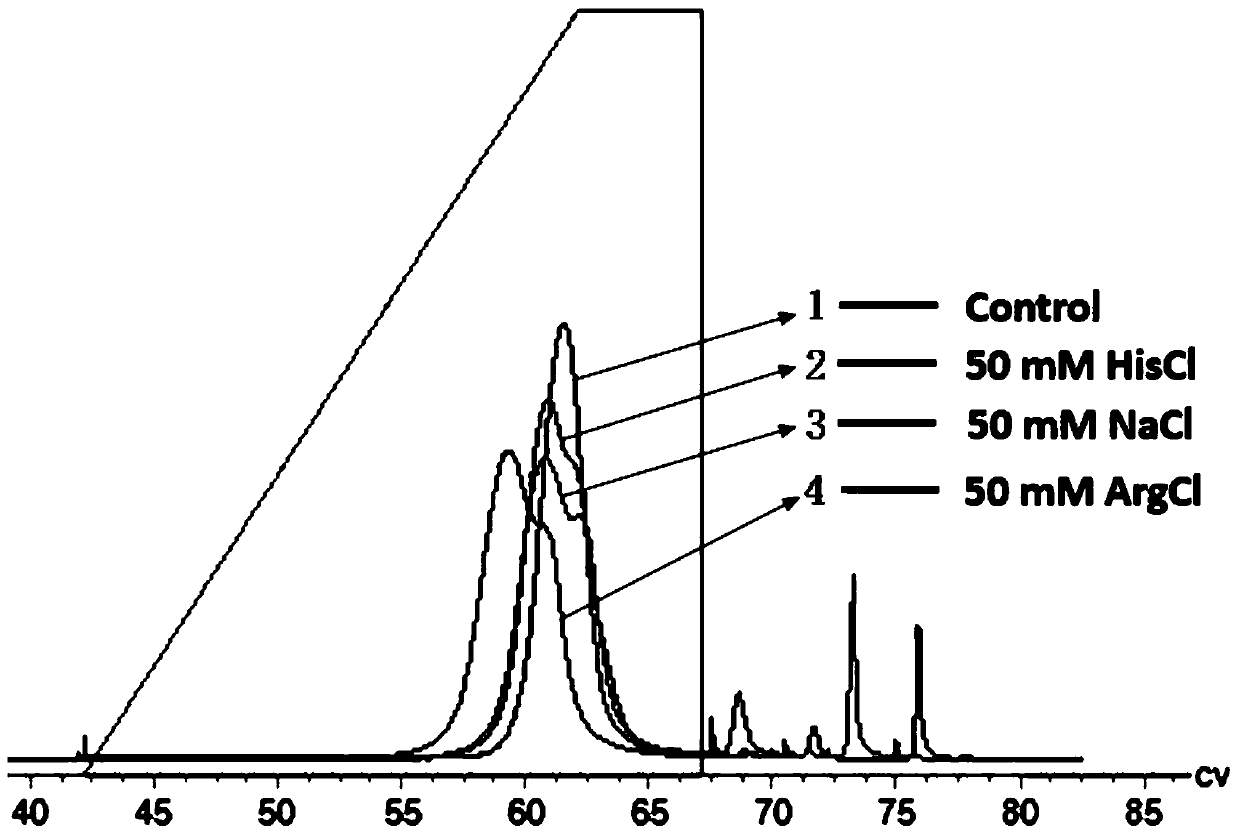
[0080] Example 1 The effect of adding salt ion additives to the elution solution in the Fc fusion protein affinity chromatography process
[0081] Use 50mM Tris-HAc, 150mM NaCl, pH 7.4 as the equilibration buffer for step 1a, step 1c, step 2a, step 5a, and step 5c; acetic acid-sodium acetate system, 50mM NaAc-HAc, 0.5M Imidazole, pH 5.5 (elution condition with salt) as the rinse 2 buffer of step 2b; 50mM NaAc-HAc, pH 5.5, as the rinse 3 buffer of step 2c; 0.1M NaOH solution as the step 1b, step 5b Disinfectant; 20% ethanol as the preservation solution for step 5d; acetic acid-sodium acetate system (50mM NaAc-HAc), with a linear gradient from pH 5.7 to 2.8, and from 0.05M NaCl, 0.05M histidine or 0.05M arginino Select the most suitable salt as an additive, and then evaluate the influence of 0.025M, 0.05M and 0.1M sodium chloride, histidine or arginine in the elution buffer on the resolution, and screen the most suitable elution salt Concentration, and finally the most suitable el...
Embodiment 2
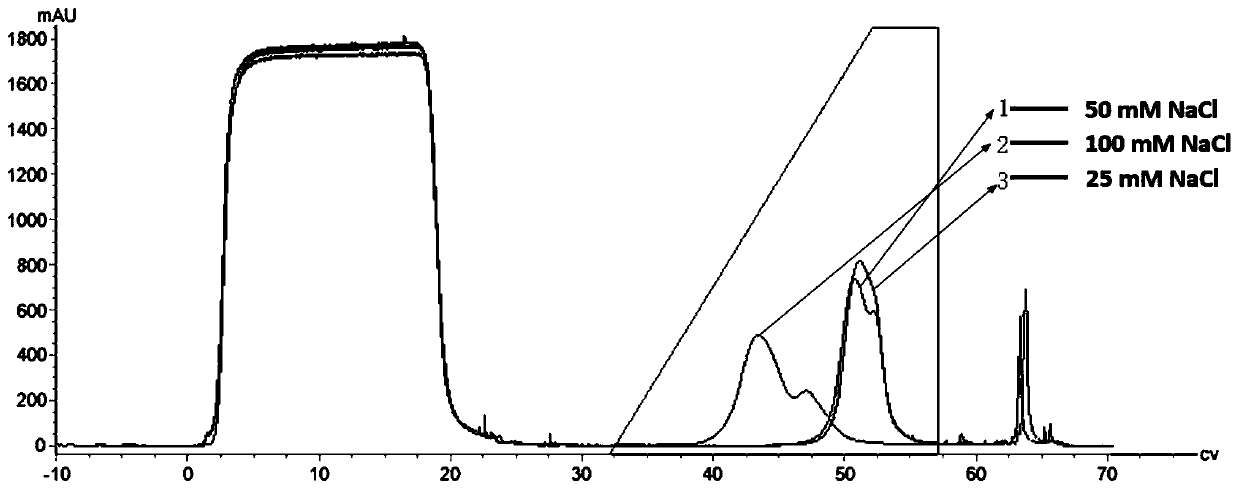
[0092] Example 2 The effect of increasing the pH value of the affinity eluate in the process of antibody protein affinity chromatography
[0093] Use 50mM Tris-HAc, 150mM NaCl, pH 7.4 as the equilibration buffer for step 1a, step 1c, step 2a, step 5a, step 5c; acetic acid-sodium acetate system, 50mM NaAc-HAc, 1M NaCl, pH 5.5 (For elution conditions without salt, use 0.5M His-HCL for 15L) as the wash 2 buffer of step 2b; 50mM NaAc-HAc, pH 5.5, as the wash 3 buffer of step 2c; 0.1M NaOH solution is used as the disinfectant of step 1b and step 5b; 20% ethanol is used as the preservation solution of step 5d; acetic acid-sodium acetate system (50mM NaAc-HAc), with a linear gradient from pH 5.7 to 2.8, and then screened by one step elution The most suitable elution conditions, and the development results are verified through 15L production. The protein A column (GEMabselect SuRe LX) was used for loading, and the loading capacity was 20-45mg / mL.
[0094] Rinse the column with 2-5 column...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com