Aryl uracil compounds, preparation method thereof and pesticide composition
A technology of aryl uracils and compounds, which is applied in the field of aryl uracil compounds and their preparation methods and pesticide compositions, can solve the problems of adverse effects on crop growth, narrow weed removal range, and poor killing effect, and achieve breastfeeding Low animal toxicity, strong herbicidal effect, and good control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] The preparation method of the aryl uracil compounds shown in compound number 4 in table 1:
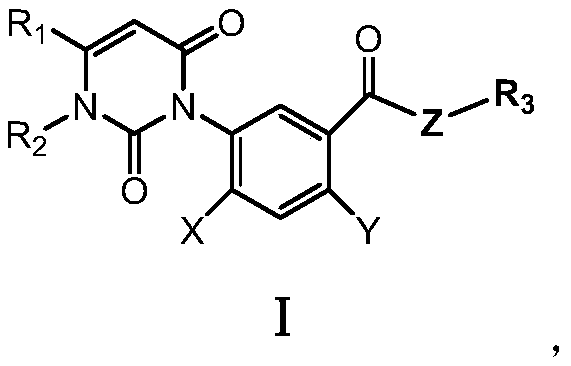
[0086] The aryluracil compound shown in the compound number 4 is shown in formula (1),
[0087] Its preparation process includes:
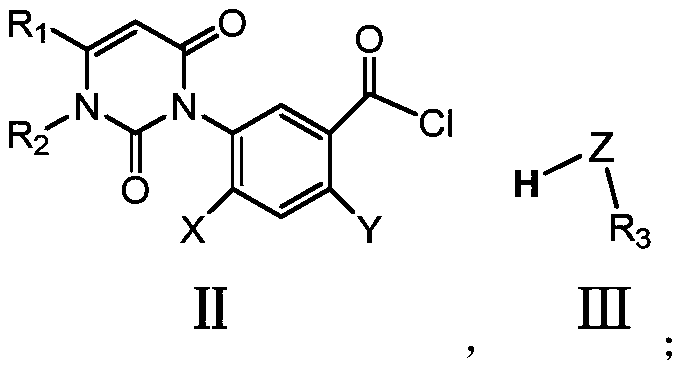
[0088] (1) 2-chloro-4-fluoro-5-(1,2,3,6-tetrahydro-2,6-dioxo-4-trifluoromethylpyrimidin-1-yl)benzoic acid (1.0g , 0.0026mol) into thionyl chloride, heated to reflux at room temperature for 6h, and spin-dried to obtain the target product 2-chloro-4-fluoro-5-(1,2,3,6-tetrahydro-2,6-di Oxy-4-trifluoromethylpyrimidin-1-yl)benzoyl chloride (0.8387 g, 0.0022 mol), 84% yield. The NMR hydrogen of the 2-chloro-4-fluoro-5-(1,2,3,6-tetrahydro-2,6-dioxo-4-trifluoromethylpyrimidin-1-yl)benzoyl chloride The spectrum is characterized by: 1 H-NMR (600MHz, DMSO), the data is as follows, δ: 8.544 (d, J = 7.6Hz, 1H, Ar), 8.072 (d, J = 9.6Hz, 1H, Ar), 6.876 (s, 1H, CH) , 3.984(s,3H,-CH 3 ). Take 2-chloro-4-fluoro-5-(1,2,3,6-tetrahydro-2,6-dioxo-4-trifluorometh...
Embodiment 2
[0093] The preparation method of the aryl uracil compounds shown in compound number 8 in table 1:
[0094] The aryluracil compound shown in the compound number 8 is shown in formula (2),
[0095] Its preparation method includes:
[0096] (1) 2-chloro-4-fluoro-5-(1,2,3,6-tetrahydro-2,6-dioxo-4-trifluoromethylpyrimidin-1-yl)benzoic acid (1.0g , 0.0026mol) into thionyl chloride, heated to reflux at room temperature for 6h, and spin-dried to obtain the target product 2-chloro-4-fluoro-5-(1,2,3,6-tetrahydro-2,6-di Oxy-4-trifluoromethylpyrimidin-1-yl)benzoyl chloride (0.8387 g, 0.0022 mol), 84% yield. The NMR hydrogen of the 2-chloro-4-fluoro-5-(1,2,3,6-tetrahydro-2,6-dioxo-4-trifluoromethylpyrimidin-1-yl)benzoyl chloride The spectrum is characterized by: 1H-NMR (400MHz, DMSO), the data is as follows, δ: 8.544 (d, J = 7.6Hz, 1H, Ar), 8.072 (d, J = 9.6Hz, 1H, Ar), 6.876 (s, 1H,CH), 3.984(s,3H,-CH 3 ).
[0097] (2) Take 2-nitroethanol (0.0911g, 0.0010mol), 2-chloro-4-fluoro-5-...
Embodiment 3
[0100] The preparation method of the aryl uracil compounds shown in compound number 12 in table 1:
[0101] The aryluracil compound shown in the compound number 12 is shown in formula (3),
[0102] Its preparation method includes:
[0103] (1) Take 2-chloro-4-fluoro-5-(1,2,3,6-tetrahydro-2,6-dioxo-4-trifluoromethylpyrimidin-1-yl)benzoic acid (0.366g , 1mmol) was mixed with 3-aminopropanol (0.0751g, 1mmol), methanesulfonic acid (1mL, 15mmol) and alumina (0.27g, 3mmol) were added as a catalyst, heated to 80°C, and refluxed for 1h.
[0104] (2) Add ethyl acetate or dichloromethane to the crude product obtained in step (1), filter, wash three times with saturated sodium chloride and saturated sodium bicarbonate solution (1:1), separate liquids, and use anhydrous chlorinated Calcium was dried, filtered, and spin-dried to obtain a light yellow powdery solid (0.06g, 0.00014mol, yield 14.2% as shown in formula (3).
[0105] H NMR spectrum characterization: 1 H-NMR (400MHz, CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com