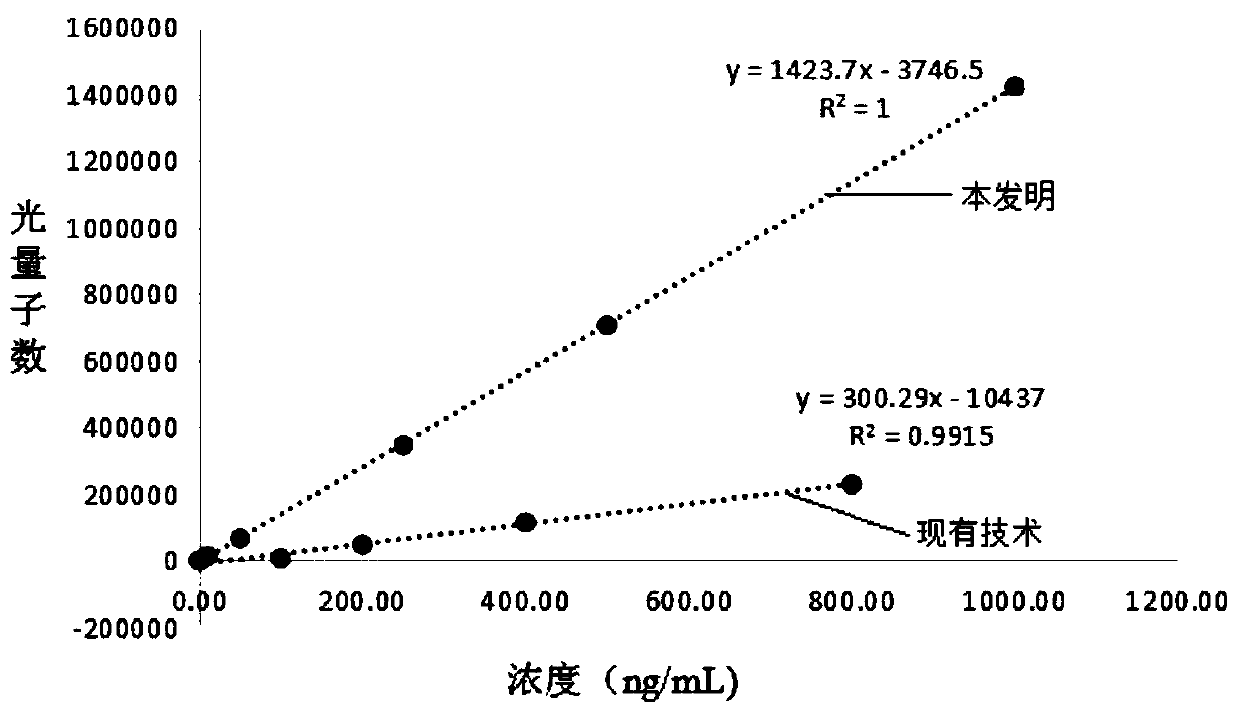
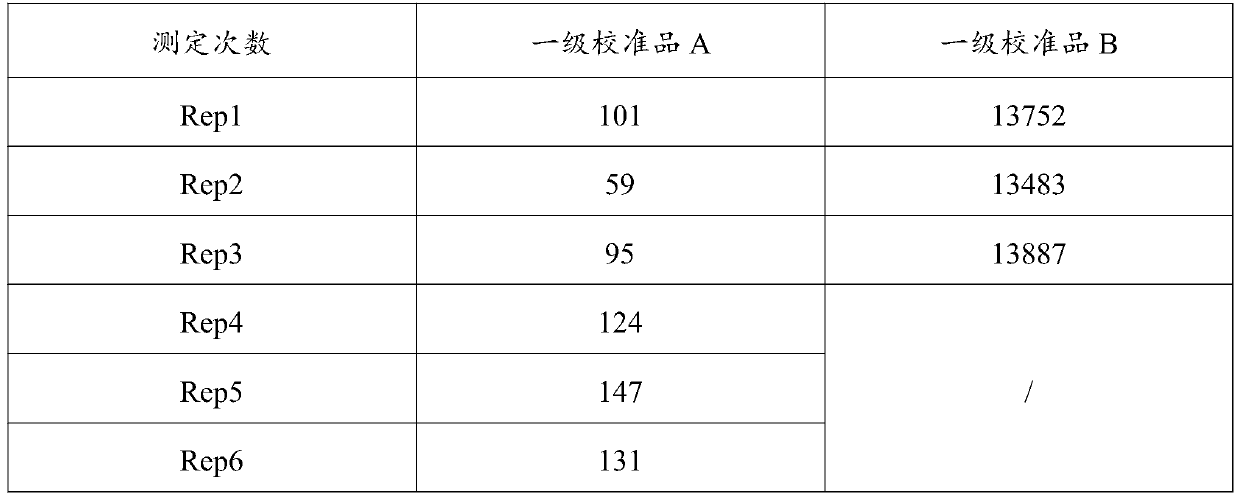
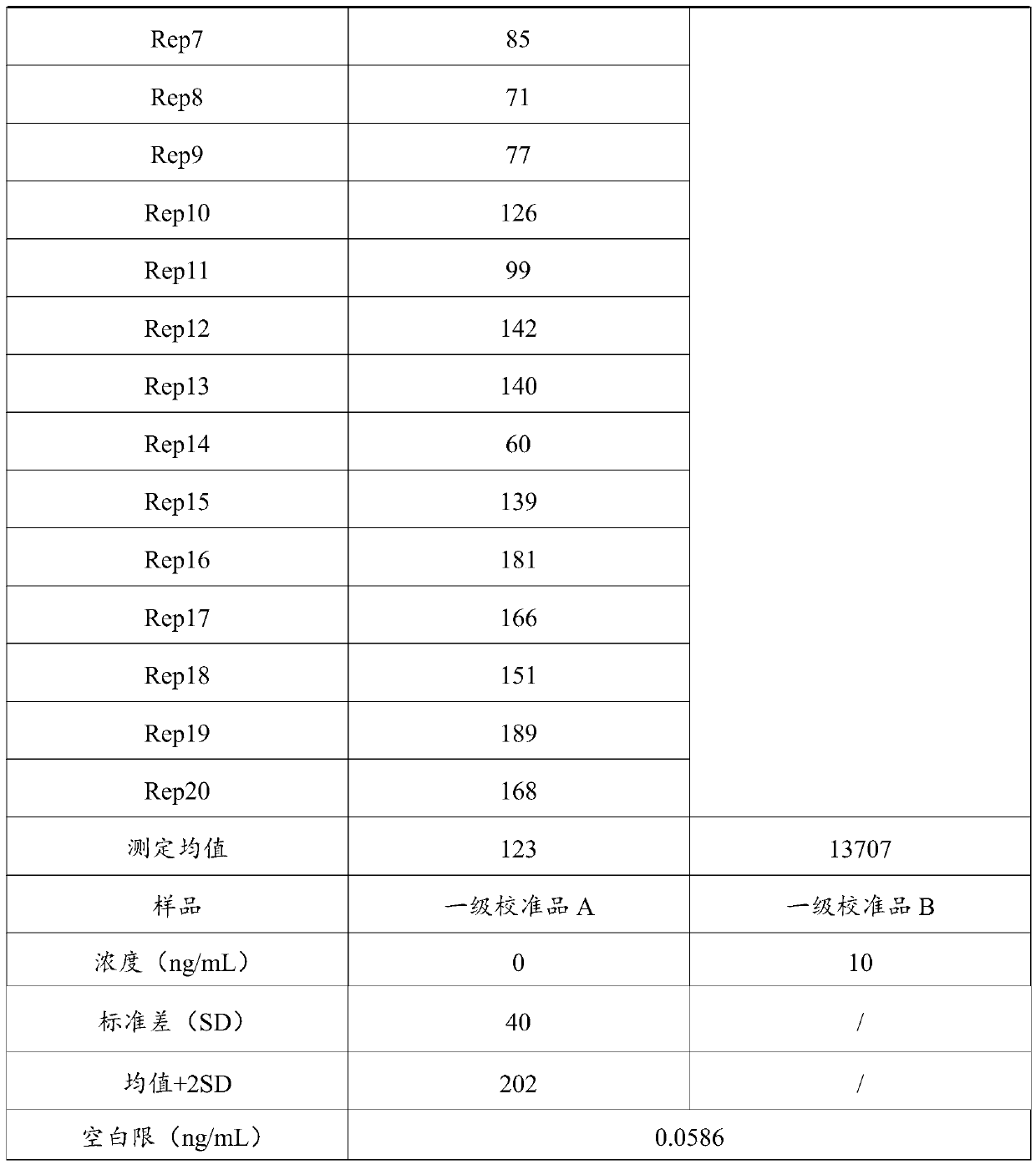
Vascular endothelial growth factor chemiluminescence immunoassay kit and preparation method thereof
A chemiluminescent immunoassay and detection kit technology, which is applied in the field of kits, can solve the problems of being greatly affected by human factors, low degree of automation, and long detection time, and achieve unattended, high degree of automation, and short detection time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The present invention also provides a preparation method of the chemiluminescent immunoassay kit for vascular endothelial growth factor, comprising the following steps:
[0046] Step 1, R1 reagent preparation
[0047] After mixing the streptavidin magnetic particle solution and TBST solution, place it on a magnetic separator until the supernatant is free of turbidity, discard the supernatant, keep the magnetic particles, wash with buffer Ⅰ and put them in buffer Ⅰ Prepare the solid-phase reagent with the required mass percentage concentration, which is the R1 reagent, and store at 2-8°C;
[0048] Wherein, the concentration of the streptavidin magnetic particle solution is preferably 50-100 mg / ml, and the source is commercially available, such as selected from Agilent Corporation, product number PL6827-1006; the volume ratio of the streptavidin magnetic particle solution to the TBST solution Preferably (0.5~1):(5~10); the mixing time is preferably 10~15min; generally re...
Embodiment 1
[0062] Step 1, R1 reagent preparation
[0063] Take 0.5mL (50mg) of streptavidin magnetic particle solution with a concentration of 100mg / mL, add 10mL of TBST solution and mix well for 10min, place on a magnetic separator until the supernatant is free of turbidity, discard the supernatant, and keep the magnetic Particles, after repeated washing with buffer Ⅰ for 3 times, make a solid-phase reagent with a mass percentage concentration of 0.05% magnetic particles in buffer Ⅰ, which is the R1 reagent, and store at 2-8°C. Buffer Ⅰ contains 50mM MES , 0.05% Tween and 0.05% Proclin300, pH6.5 buffer.
[0064] Step 2, R2 reagent preparation
[0065] Put 250μg of antibody into a centrifuge tube, make sure that the antibody is at the bottom of the centrifuge tube (centrifuge at room temperature for 20s), then add PBS buffer solution, mix well, add 5μl 2mg / mL acridinium ester DMF solution after mixing, and centrifuge Centrifuge for 0.5 min at room temperature. Seal the centrifuge tube...
Embodiment 2
[0069] Step 1, R1 reagent preparation
[0070] Take 0.72 milliliters (72 mg) of streptavidin magnetic particle solution with a concentration of 100 mg / ml, add 15 mL of TBST solution and mix well for 15 minutes, then place it on a magnetic separator until the supernatant is free of turbidity, discard the supernatant, and keep Take the magnetic particles, wash with buffer Ⅰ repeatedly for 3 times, and then prepare a solid-phase reagent with a mass percentage concentration of 0.072% magnetic particles in buffer Ⅰ, which is the R1 reagent, and store at 2-8°C. Buffer Ⅰ contains 100 mMPBS, 0.1% Tween-20 and 0.1% Proclin300, pH 7.2 buffer.
[0071] Step 2, R2 reagent preparation
[0072] Put 500μg of antibody into a centrifuge tube, make sure that the antibody is at the bottom of the centrifuge tube (centrifuge at room temperature for 30s), then add TRIS washing solution, mix well, add 15μl 2.5mg / mL acridinium ester DMF solution after mixing, use The centrifuge was centrifuged at roo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com