Serum-free umbilical cord mesenchymal stem cell composition and application thereof
A mesenchymal stem cell and composition technology, applied in the field of umbilical cord mesenchymal stem cell composition, can solve the problems of joint function decline, poor treatment effect of articular cartilage injury, limited source of cartilage particles, etc., to achieve easy operation and avoid surgical risks Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Isolation, purification and culture of umbilical cord mesenchymal stem cells
[0067] (1) Wash the umbilical cord tissue (remove blood cells): add an equal amount of normal saline to the centrifuge tube containing the umbilical cord, tighten the cap, shake for 3 minutes to fully wash the umbilical cord tissue, then stand still for 3-5 minutes to separate the different phases, and suck off the lower layer Water phase; repeat the above operation three times until the lower layer is relatively clear.
[0068] (2) Ultrasonic microwave pulverization: After absorbing and discarding the normal saline, add preheated DMEM equal to the volume of the umbilical cord, put it in a constant temperature microwave pulverizer, and conduct ultrasonic microwave pulverization at 37°C.
[0069] (3) Collect the precipitate: after digestion, centrifuge at 2000rpm for 10min, discard the digested umbilical cord in the upper layer, collect the bottom layer of the two tubes into a new centrifuge t...
Embodiment 2
[0074] Identification of umbilical cord mesenchymal stem cells
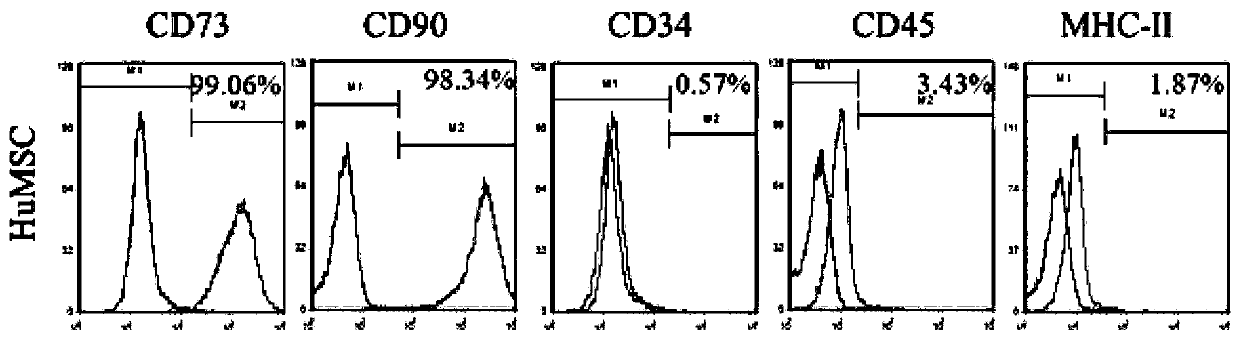
[0075] The P2 generation umbilical cord mesenchymal stem cells cultured in Example 1 were centrifuged and resuspended with 15% human albumin solution and 0.9% sodium chloride solution, wherein the volume of the human albumin solution and the sodium chloride solution The ratio was 0.1:1; the cell concentration was adjusted to l×10 after cell counting 8 / L, respectively reacted with human anti-CD73, CD90, CD34, CD45, MHC-II monoclonal antibodies at room temperature for 30 minutes, resuspended the cells in PBS, and detected them by flow cytometry. The test results are shown in the table below and figure 1 . The cell surface antigen marker expression of umbilical cord mesenchymal stem cells was analyzed by flow cytometry, and the cells were of high purity.
[0076] surface antigen CD73 CD90 CD34 CD45 MHC-II result 99.06% 98.34% 0.57% 3.43% 1.87%
Embodiment 3
[0078] Umbilical cord mesenchymal stem cells osteogenic, adipogenic, chondrogenic ability test
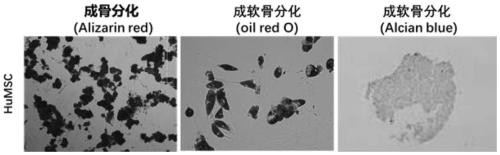
[0079] The cells cultured in Example 1 were used as the group of the present invention, and the osteogenic, adipogenic and chondrogenic abilities were tested. Alizarin Red S, Oil Red O, and Alcian blue staining showed that the cells cultured in Example 1 had the ability to differentiate into bone, fat, and cartilage in vitro after 3-4 weeks of culture. see results figure 2 , reflecting the in vitro differentiation ability of umbilical cord mesenchymal stem cells
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com