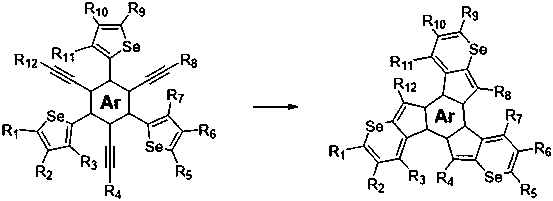
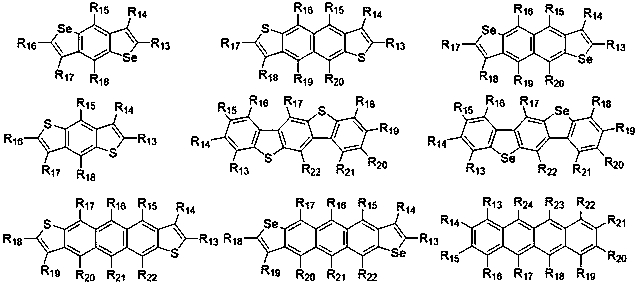
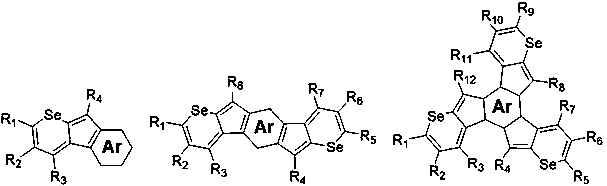
Near infrared organic photoelectric material containing cyclopentadiene selenium furan, and preparation method thereof
A cyclopentadiene and near-infrared technology, applied in organic dyes, organic chemistry, chemical instruments and methods, etc., can solve the problems of lack of synthesis methods, etc., and achieve the effects of broad application prospects, high yield, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the preparation of compound 1
[0022]
[0023] Under nitrogen atmosphere, weigh 344mg (1.0mmol) precursor 1a and 30mg (0.01mmol) PtCl 2 , dissolved in 30mL toluene, heated to 80°C, and reacted for 3 hours. After the reaction, the toluene in the reaction system was removed, and the crude product was separated and purified by silica gel column chromatography to obtain 265mg of compound 1 with a yield of 77%. The lowest half-wave potential of oxidation was 0.28V. , and its electrochemical performance remained unchanged.
Embodiment 2
[0024] Embodiment 2: the preparation of compound 2
[0025]
[0026] In an argon atmosphere, 554 mg (1.0 mmol) of precursor 2a and 60 mg (0.02 mmol) of PtCl 2 (PPh 3 ) 2 Disperse in 50mL of toluene, heat to 85°C, and react for 5 hours. After the reaction, the toluene in the reaction system was removed, and the crude product was separated and purified by column chromatography to obtain 450mg of compound 2 with a yield of 81%. The lowest half-wave potential of oxidation was -0.03V, after 100 cycles of redox cyclic voltammetry scanning , and its electrochemical performance remained unchanged.
Embodiment 3
[0027] Embodiment 3: the preparation of compound 3
[0028]
[0029] Under argon protection, 876 mg (1.0 mmol) of precursor 3a and 90 mg (0.03 mmol) of platinum chloride PtCl 2 Disperse in 70mL of toluene, heat to 95°C, and react for 10 hours. After the reaction was completed, the toluene in the reaction system was removed, and the crude product was separated and purified by silica gel column chromatography to obtain 385mg of compound 3 with a yield of 44%. The lowest half-wave potential of oxidation was -0.04V, after 50 cycles of redox cyclic voltammetry scanning After that, its electrochemical performance remained unchanged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com