Human target complement inhibitor protein mcr2-fh and its application
A complement inhibitor and fusion protein technology, applied in the field of fusion proteins, can solve the problems of increased protection rate in animal protection experiments, and the inhibition effect is not as good as in vitro inhibition experiments, etc., and achieves excellent anti-adhesion/anti-inflammatory targeted inhibition effect, crescent / Necrosis improvement, excellent application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
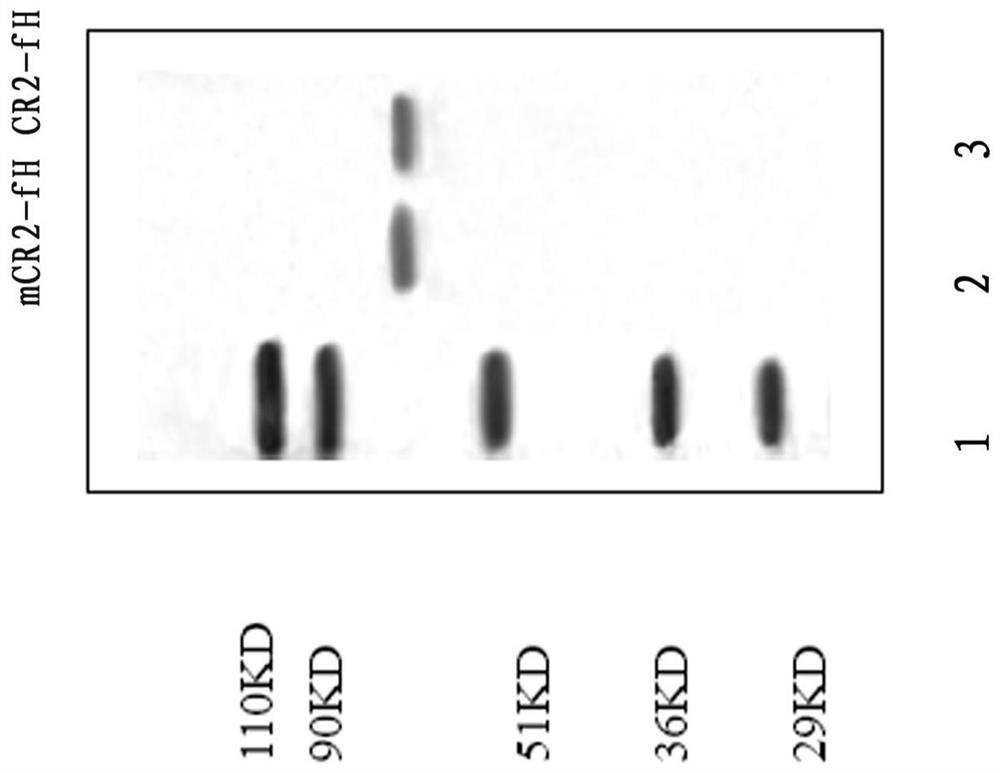
[0033] Example 1. Preparation of CR2 mutant (mCR2)-fH fusion protein
[0034] 1 Materials The expression vector was pEE14.1 (Lonza biologics); CHO cells were used for protein expression, and the culture medium was DMEM containing 10% fetal bovine serum, purchased from Invitrogen Company. Mouse anti-fH mAbs 1H4 and 1A10, mouse anti-human CR2 mAb171, anti-goat erythrocyte IgM and all secondary antibodies were purchased from Sigma.
[0035] 2 methods
[0036] 2.1 Preparation of antiserum of rabbit anti-CHO cell membrane and human fH according to the literature Harlow, E., and Lane, D. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory. Cold Spring Harbor, New York, USA. 1988:726. method to obtain.
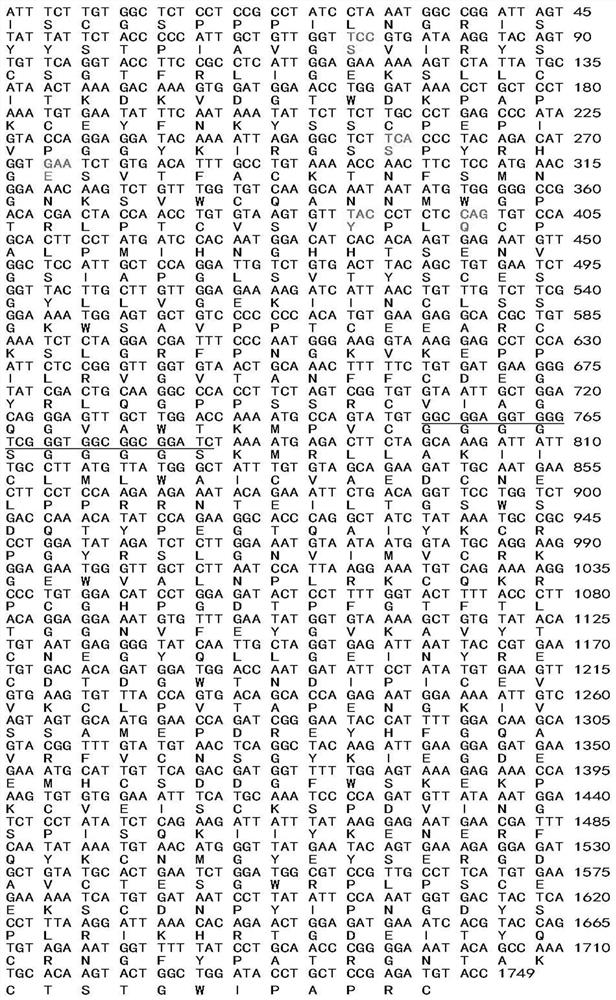
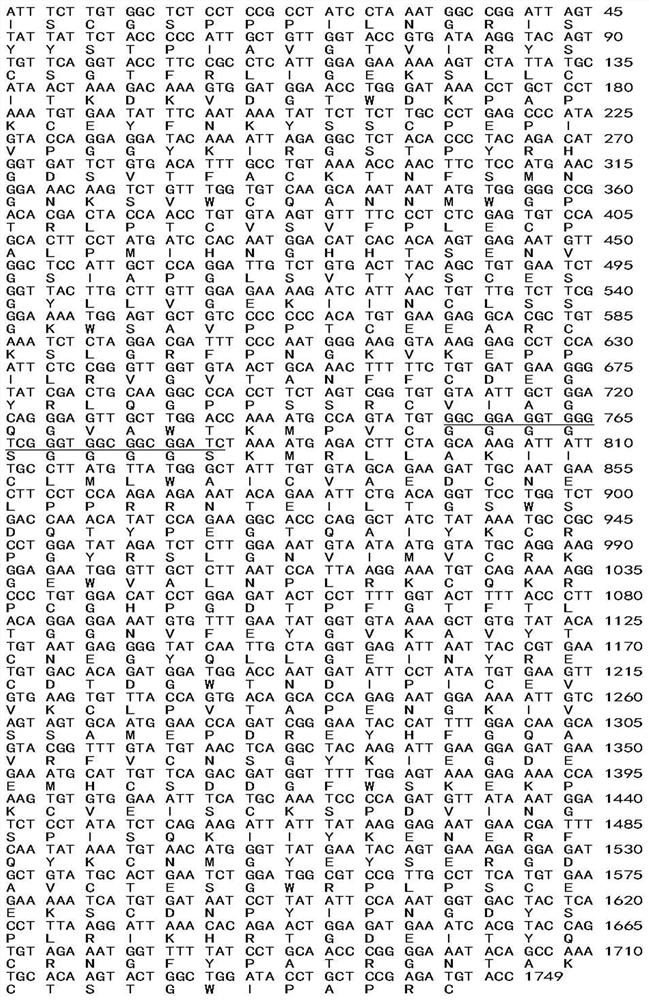
[0037] 2.2 Construction of expression recombinant and protein expression The cDNA structure gene is formed by connecting the four N-terminal SCR units encoding CR2 and the sequence encoding the extracellular region of fH. The complement inhibitor sequence is the 322 ami...
Embodiment 2
[0044] Example 2. Kinetic analysis of interaction between CR2 fusion protein and C3 ligand
[0045]Kinetic analysis of the interaction of the CR2 fusion protein with biotin-labeled C3dg (C3dg-biotin) was detected with a Surface Cytoplasmic Resonance (SPR) detection system (BIAcore 3000 instrument). Human C3dg-biotin (Guthridge, J.M., et al. Structural studies insolution of the recombinant N-terminal pair of short consensus / complementrepeat domains of complement receptor type 2 (CR2 / CD21) and interactions with its ligand C3dg. Biochemistry. 2001, 40(20): 5931–5941.) were injected onto the BIAcore streptavidin sensor chip at a rate of 2 μL / min for 20 min, and the buffer was 0.5× PBS (pH 7.4) (containing 0.5 g / L Tween20). The SPR signal acquired from the captured C3dg yielded BIAcore response units (range 250 to 500). The group without fusion protein was used as a control. After washing with 0.5×PBST (0.5g / LTween20) at 25°C at a flow rate of 25μL / min, the affinity of the CR2 ...
Embodiment 3
[0049] Example 3. Complement lysis assay
[0050] To determine the inhibitory activity against complement, 60%-80% confluent CHO cells were split with EDTA, washed twice with DMEM, and then resuspended in DMEM to a final concentration of 10 6 cells / mL. 100 mL / L rabbit anti-CHO cell membrane antiserum was added to the cell suspension, and the cells were sensitized for 30 min at 4°C. Antiserum was then discarded and cells were resuspended in NHS diluted in DMEM in a final volume of 50 μL or 100 μL. The cells were incubated at 37°C for 60 min, and finally the cell viability was measured by placental blue staining exclusion method (both live and dead cells were counted). The recombinant fusion protein was diluted with DEME and added to NHS and then to the CHO cell suspension. The final concentration was based on the lysis of control CHO cells in which 100 g / L NHS resulted in approximately 90% antibody sensitization. The complement-mediated inhibition of erythrocyte lysis assay...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com