Deamidated gliadin polypeptide recombinant antigen, recombinant antigen expression gene, recombinant expression vector, preparation method and application thereof
A protein polypeptide, recombinant antigen technology, applied in the direction of fusion polypeptide, chemical instruments and methods, recombinant DNA technology, etc., can solve the problem of serum false negative
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of the above-mentioned deamidated gliadin polypeptide recombinant antigen according to an embodiment of the present invention comprises the following steps: obtaining the nucleotide sequence corresponding to the amino acid sequence of the above-mentioned deamidated gliadin polypeptide recombinant antigen, and passing the double-enzyme cutting and ligating into the expression vector to obtain a recombinant expression vector, transfer the recombinant expression vector into a host for expression, and then separate and purify the expression product to obtain a deamidated gliadin polypeptide recombinant antigen.
[0034] In a specific example, the nucleotide sequence corresponding to the amino acid sequence of the above-mentioned deamidated gliadin polypeptide recombinant antigen can be obtained by gene synthesis, and the host can be selected according to the type of expression vector, such as Escherichia coli BL21 (DE3) strain Wait.
[0035] The prese...
Embodiment 1
[0037] 1. Preparation of recombinant antigen of deamidated gliadin polypeptide
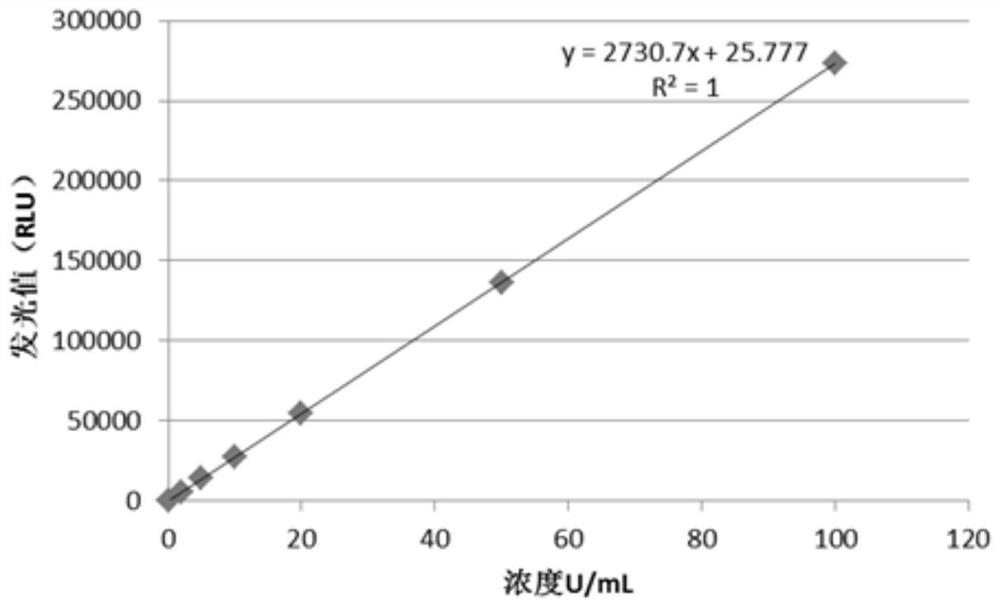
[0038] Select DGP dominant epitope peptides, the amino acid sequences are respectively SEQ ID No.1 (named as DGP-1 peptide) and SEQID No.2 (named as DGP-2 peptide), and the amino acid sequence of IL-15 protein is SEQ ID No.3, the amino acid sequence of the hexahistidine tag is SEQ ID No.4, the amino acid sequence of the Flag tag is SEQ ID No.5, and the amino acid sequence of the connecting peptide is SEQ ID No.6. The hexahistidine tag, DGP-1 peptide, interleukin 15, DGP-2 peptide and Flag tag are all connected by connecting peptides and fused into DGP recombinant antigen, the sequence of which is SEQ ID No.7. The DNA of the DGP recombinant antigen was obtained through gene synthesis (General Biosystems (Anhui) Co., Ltd.), and the sequence is SEQ ID No.8. The DNA of the DGP recombinant antigen has an Nde I site upstream and a Hind III site downstream. The DNA was digested with the corresponding r...
Embodiment 2
[0063] The method of this example is basically the same as that of Example 1, the only difference is that pET-28a(+) is selected as the expression vector. Correspondingly, the sensitivity of the prepared detection kit was 0.8 U / mL, the intra-assay and inter-assay differences of the kit were CV=3.65%, CV=4.87%, and the interference met the document standard of NCCLS.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com