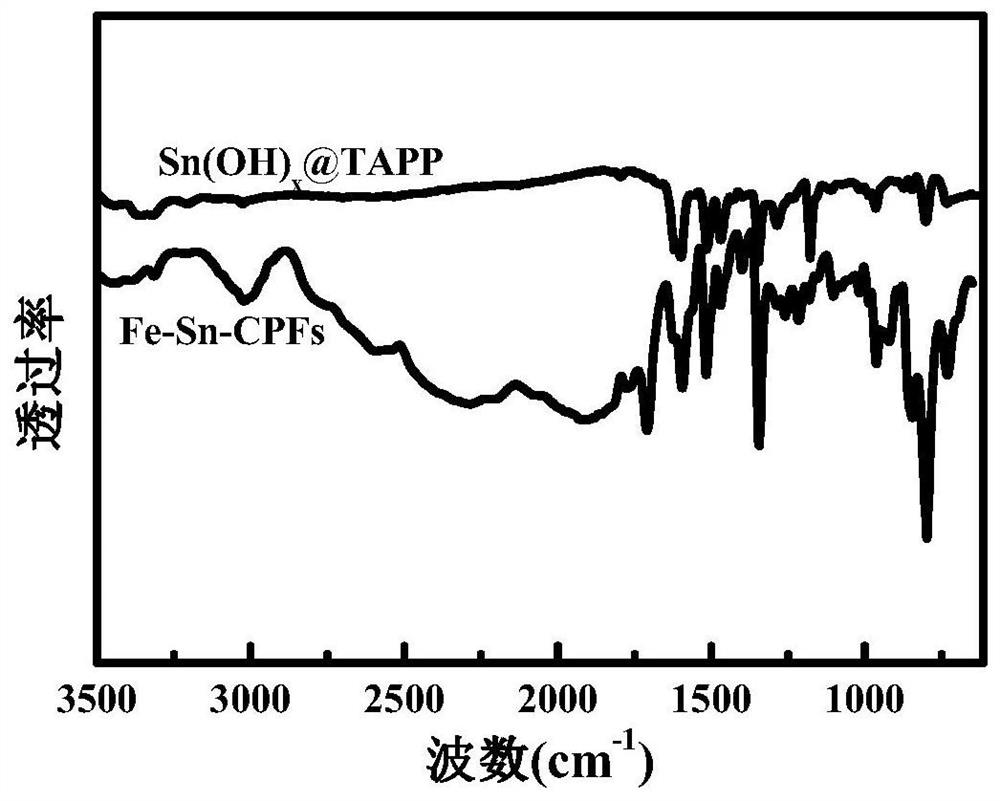
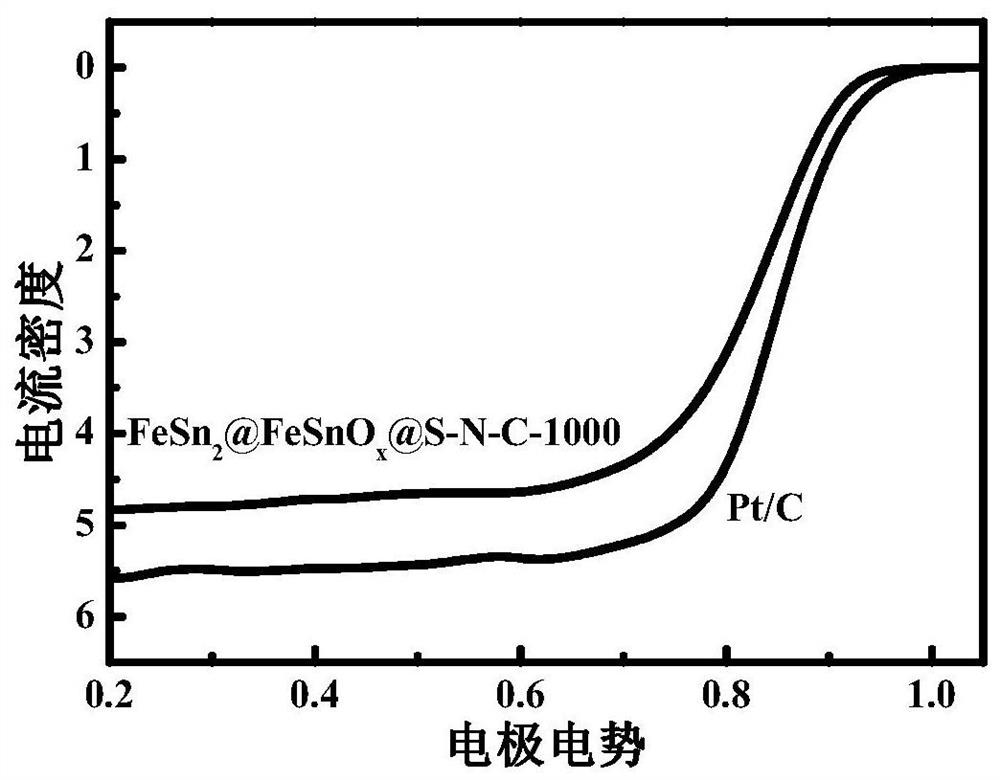
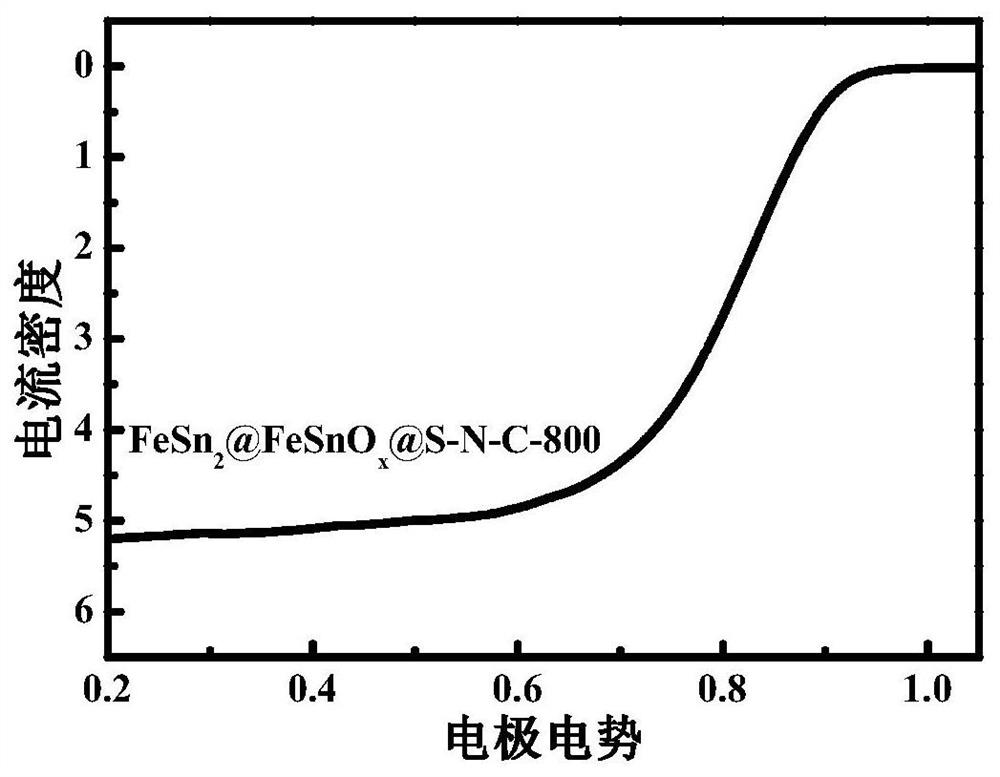
Iron-tin alloy-supported sulfur-nitrogen co-doped carbon electrocatalyst and preparation method
A technology of iron-tin alloy and electrocatalyst, which is applied in fuel cell-type half-cells and secondary battery-type half-cells, circuits, electrical components, etc., to achieve improved stability, high open circuit potential, and uniform distribution of active sites Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] a. Preparation of 5,10,15,20-tetra(nitro)phenylporphyrin
[0032] Add 0.1 mole of p-nitrobenzaldehyde to 100 mL of boiling propionic acid (analytically pure) and stir for 30 min, add dropwise 10 mL of propionic acid containing 0.2 mole of pyrrole, reflux for 1 h at a temperature of 130 ° C, and use the filtered product after the reaction Wash thoroughly with deionized water and dry under vacuum. Afterwards, reflux washing with pyridine, cooling overnight, suction filtration, washing with acetone (analytical pure) until the filtrate is colorless, and after vacuum drying, it is 5,10,15,20-tetrakis(nitro)phenylporphyrin (TNPP).
[0033] b. Preparation of doped Sn(OH) x 5,10,15,20-tetra(amino)phenylporphyrin
[0034] Under the protection of nitrogen, take 0.1 mole of 5,10,15,20-tetra(nitro)phenylporphyrin synthesized above and dissolve 0.8 mole of SnCl in 100 mL of concentrated hydrochloric acid (analytical pure) 2 2H 2 O dissolved in 25mL of concentrated hydrochloric a...
Embodiment 2
[0043] a. Preparation of 5,10,15,20-tetra(nitro)phenylporphyrin
[0044] Add 0.1 mole of p-nitrobenzaldehyde into 120 mL of boiling propionic acid and stir for 30 min, add dropwise 10 mL of propionic acid containing 0.3 mole of pyrrole, and reflux at 130°C for 1 h. Wash and dry in vacuo. Afterwards, reflux washing with pyridine, cooling overnight, suction filtration, washing with acetone (analytical pure) until the filtrate is colorless, and after vacuum drying, it is 5,10,15,20-tetrakis(nitro)phenylporphyrin (TNPP).
[0045] b. Preparation of doped Sn(OH) x 5,10,15,20-tetra(amino)phenylporphyrin
[0046] Under the protection of nitrogen, take the 0.1 mole doped Sn(OH) prepared above x 5,10,15,20-tetra(amino)phenylporphyrin and 0.9 mole SnCl 2 2H 2 O mixed, dissolved with concentrated hydrochloric acid (analytically pure), stirred at room temperature for 2.5 hours, heated to 70°C, reacted for 30 minutes, cooled in an ice-water bath after the reaction, poured 100 ml of dei...
Embodiment 3
[0053] Preparation of a.5,10,15,20-tetra(nitro)phenylporphyrin
[0054] Add 0.1 mole of p-nitrobenzaldehyde into 150 mL of boiling propionic acid and stir for 30 min, add dropwise 10 mL of propionic acid containing 0.3 mole of pyrrole, and reflux at 130°C for 1 h. Wash and dry in vacuo. Afterwards, reflux washing with pyridine, cooling overnight, suction filtration, washing with acetone (analytical pure) until the filtrate is colorless, and after vacuum drying, it is 5,10,15,20-tetrakis(nitro)phenylporphyrin (TNPP).
[0055] b. Doping Sn(OH) x Preparation of 5,10,15,20-tetra(amino)phenylporphyrin
[0056] Under the protection of nitrogen, take 0.1 mole of 5,10,15,20-tetra(nitro)phenylporphyrin synthesized above and dissolve 1.0 mole of SnCl in 100 mL of concentrated hydrochloric acid (analytical pure) 2 2H 2 O dissolved in 25mL of concentrated hydrochloric acid (analytical pure) was added dropwise to the above system, stirred at room temperature for 2.5h, heated to 70°C, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com