Preparation method of moxifloxacin hydrochloride
A technology for moxifloxacin hydrochloride and hydrochloric acid is applied in the field of preparation of moxifloxacin hydrochloride, which can solve the problems of large loss of moxifloxacin hydrochloride, low yield and liquid phase purity, and insufficient product quality, and achieves low total impurity content. , the effect of improving purity and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
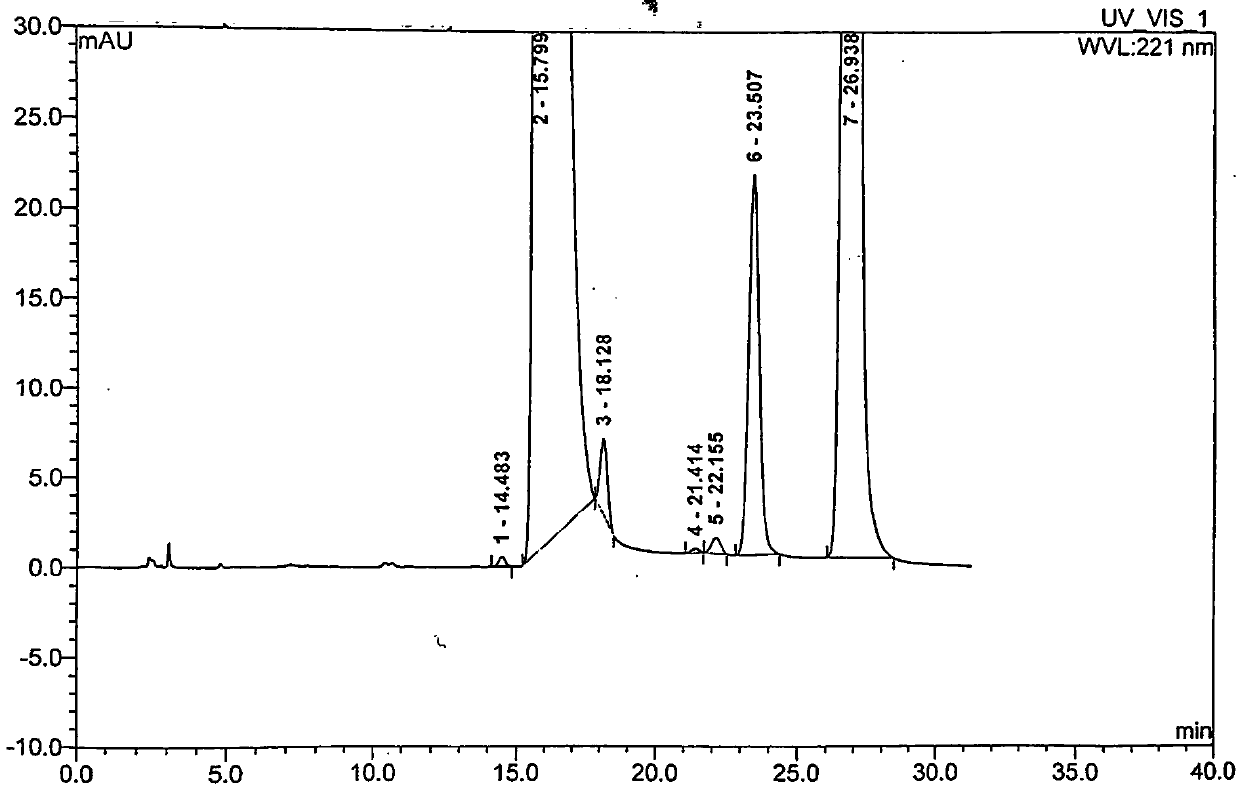
[0035] A preparation method of moxifloxacin hydrochloride, using ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate Condensate with (S,S)-2,8-diazabicyclo[4,3,0]nonane as the parent ring, then add 6N hydrochloric acid twice after the alkali hydrolysis is completed, and continuously crystallize two The second time, the crystalline product obtained in the second time was recrystallized once with ethanol to obtain moxifloxacin hydrochloride with high content and high quality. Specific steps are as follows:
[0036] (1) Add the weighed boric acid and zinc chloride into a 50L reaction tank, pump acetic anhydride into the reaction tank, heat with jacket steam under stirring, raise the temperature to 110°C-120°C, and keep the temperature for 2 hours; the heat preservation is over , lower the temperature to below 100°C, pump glacial acetic acid into the reaction tank, raise the temperature to 110°C-120°C, and keep it warm for 1 hour.
[0037] (2) Open ...
Embodiment 2
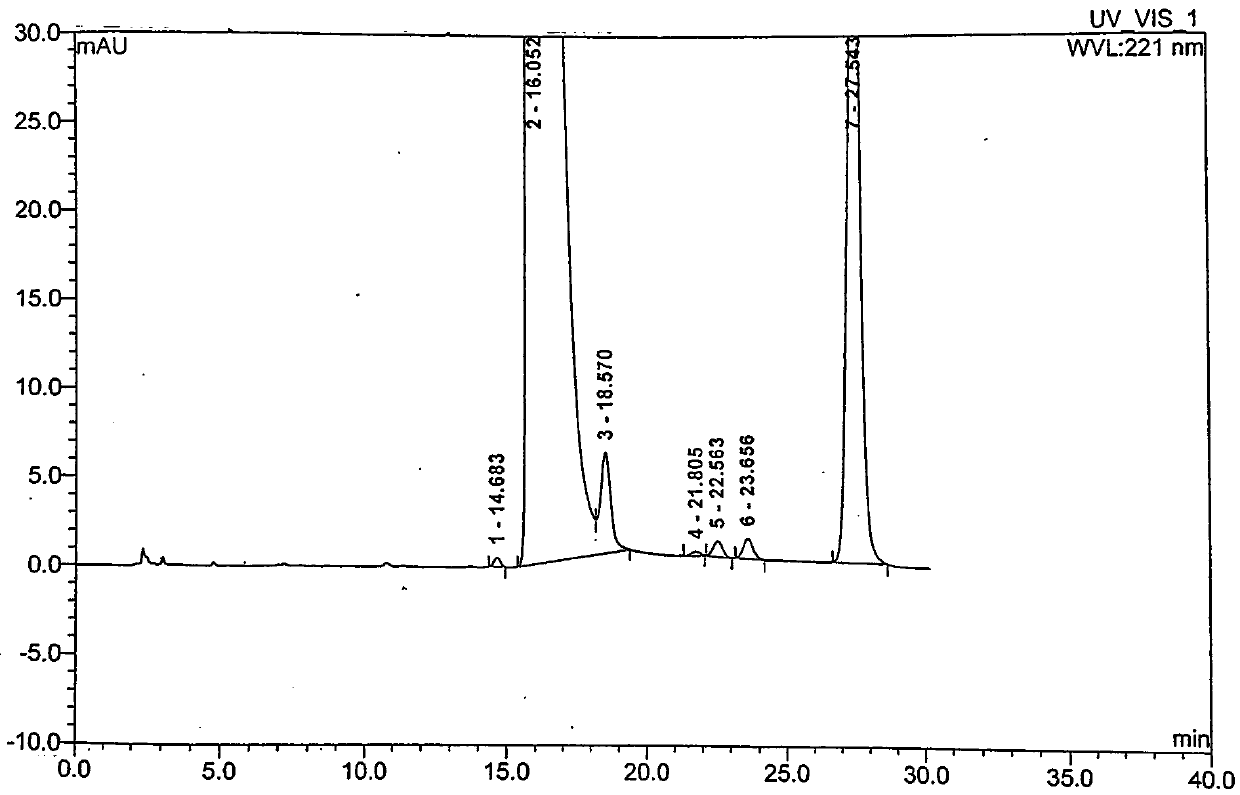
[0050] A preparation method of moxifloxacin hydrochloride, using ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate Condensate with (S,S)-2,8-diazabicyclo[4,3,0]nonane as the parent ring, then add 6N hydrochloric acid twice after the alkali hydrolysis is completed, and continuously crystallize two The second time, the crystalline product obtained in the second time was recrystallized once with ethanol to obtain moxifloxacin hydrochloride with high content and high quality. Specific steps are as follows:
[0051] (1) Add the weighed boric acid and zinc chloride into a 50L reaction tank, pump acetic anhydride into the reaction tank, heat with jacket steam under stirring, raise the temperature to 110°C-120°C, and keep the temperature for 2 hours; the heat preservation is over , lower the temperature to below 100°C, pump glacial acetic acid into the reaction tank, raise the temperature to 110°C-120°C, and keep it warm for 1 hour.
[0052] (2) Ope...
Embodiment 3
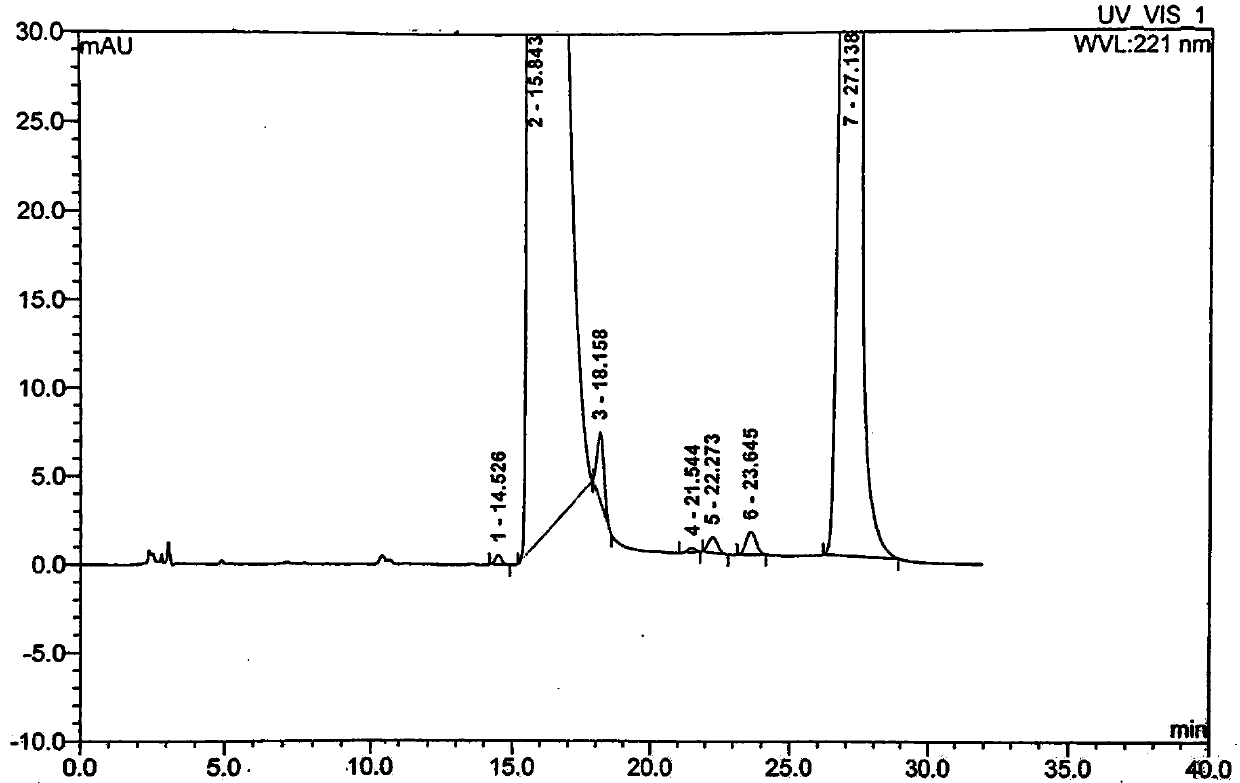
[0065] A preparation method of moxifloxacin hydrochloride, using ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate Condensate with (S,S)-2,8-diazabicyclo[4,3,0]nonane as the parent ring, then add 6N hydrochloric acid twice after the alkali hydrolysis is completed, and continuously crystallize two The second time, the crystalline product obtained in the second time was recrystallized once with ethanol to obtain moxifloxacin hydrochloride with high content and high quality. Specific steps are as follows:
[0066] (1) Add the weighed boric acid and zinc chloride into a 50L reaction tank, pump acetic anhydride into the reaction tank, heat with jacket steam under stirring, raise the temperature to 110°C-120°C, and keep the temperature for 2 hours; the heat preservation is over , lower the temperature to below 100°C, pump glacial acetic acid into the reaction tank, raise the temperature to 110°C-120°C, and keep it warm for 1 hour.
[0067] (2) Ope...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com