Salt separation evaporation crystallization and purification method
A technology of evaporative crystallization and purification method, which is applied in evaporative separation crystallization, chemical instruments and methods, crystallization separation, etc., and can solve problems such as salt pollution, water quality hazards, and failure to achieve zero discharge.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
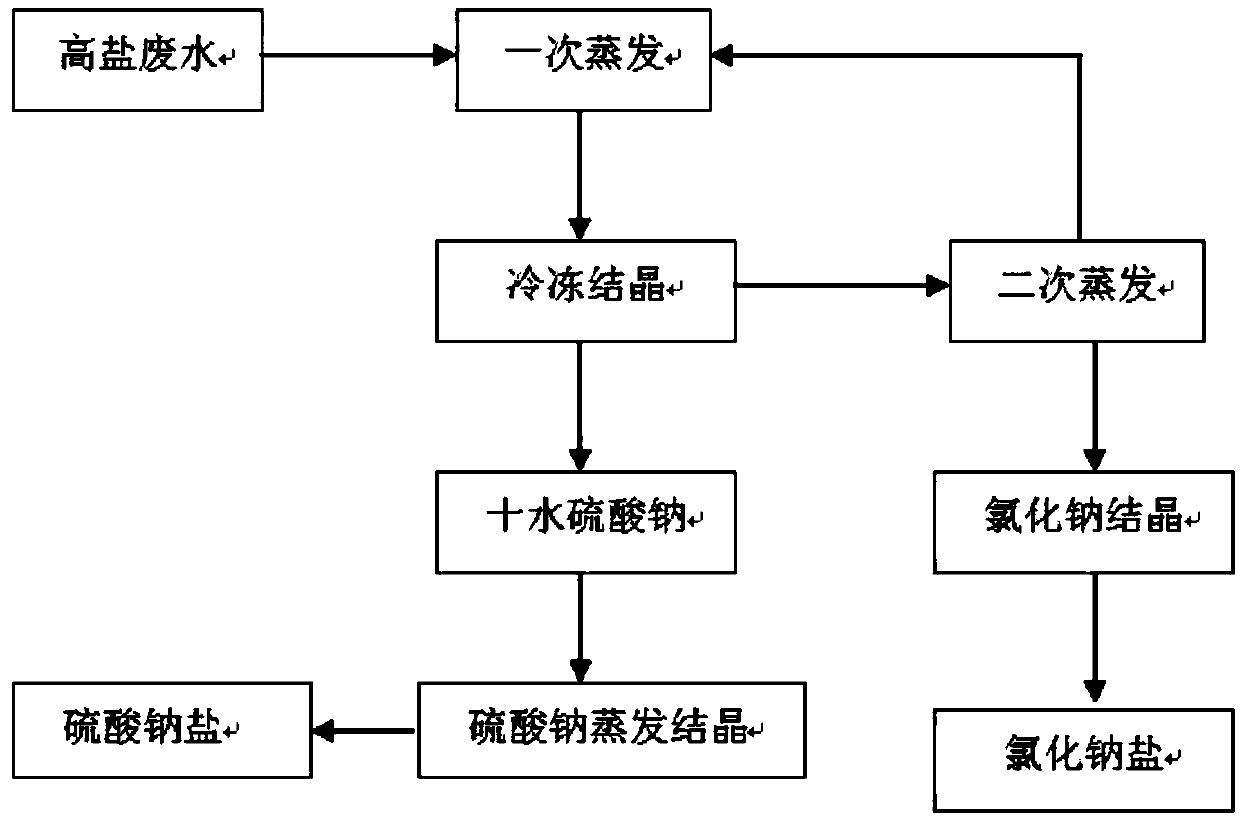
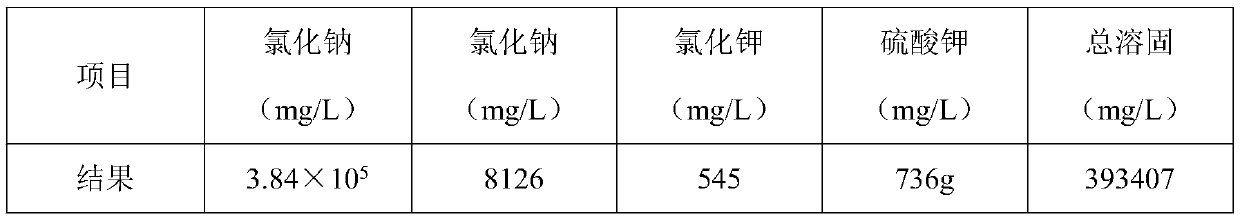
[0021] S1: Primary evaporation: change the salt content of high-salt wastewater from 0.8×10 5 mg / L concentrated to 3.2×10 5 mg / L, the temperature of one evaporation concentration is 50°C;
[0022] S2: Freeze crystallization: transport the concentrated wastewater obtained in S1 to the freeze crystallization device to precipitate Na 2 SO 4 10H 2 O crystal, realizing the separation of sodium sulfate and sodium chloride, the temperature of freezing and crystallization is 0°C;
[0023] S3: Evaporation and purification: the Na obtained by S2 2 SO 4 10H 2 O is purified by evaporation to produce sodium sulfate with a final purity of 99.3%;
[0024] S4: Secondary evaporation: The mother liquor obtained in S2 is evaporated and crystallized again to produce sodium chloride salt with a purity of 98.2%. The temperature of the second evaporation and crystallization is 80°C, and the remaining mother liquor after evaporation is returned to the primary evaporation water for recycling. ...
Embodiment 2
[0026] S1: Primary evaporation: change the salt content of high-salt wastewater from 1.2×10 5 mg / L concentrated to 2.8×10 5 mg / L, the temperature of one evaporation concentration is 100°C;
[0027] S2: Freeze crystallization: transport the concentrated wastewater obtained in S1 to the freeze crystallization device to precipitate Na 2 SO 4 10H 2 O crystal, realizing the separation of sodium sulfate and sodium chloride, the temperature of freezing and crystallization is 0°C;
[0028] S3: Evaporation and purification: the Na obtained by S2 2 SO 4 10H 2 O is purified by evaporation to produce sodium sulfate with a final purity of 99.5%;
[0029] S4: Secondary evaporation: The mother liquor obtained in S2 is evaporated and crystallized again to produce sodium chloride salt with a purity of 98.6%. The temperature of the second evaporation and crystallization is 100°C, and the remaining mother liquor after evaporation is returned to the primary evaporation water for recycling....
Embodiment 3
[0031] S1: Primary evaporation: change the salt content of high-salt wastewater from 1.0×10 5 mg / L concentrated to 3.0×10 5 mg / L, the temperature of one evaporation concentration is 80°C;
[0032] S2: Freeze crystallization: transport the concentrated wastewater obtained in S1 to the freeze crystallization device to precipitate Na 2 SO 4 10H 2 O crystal, realizing the separation of sodium sulfate and sodium chloride, the temperature of freezing and crystallization is 0°C;
[0033] S3: Evaporation and purification: the Na obtained by S2 2 SO 4 10H 2 O is purified by evaporation to produce sodium sulfate with a final purity of 99.3%;
[0034] S4: Secondary evaporation: The mother liquor obtained in S2 is evaporated and crystallized again to produce sodium chloride salt with a purity of 98.5%. The temperature of the second evaporation and crystallization is 90°C, and the remaining mother liquor after evaporation is returned to the primary evaporation water for recycling. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com