6-glucose-6-phosphate dehydrogenase mutant and application thereof in preparing detection reagent
A technology for glucose phosphate and detection reagents, which is applied in the field of enzyme 6-phosphate glucose dehydrogenase and its application in detection kits, and can solve the problems of cumbersome operation, long time-consuming and short validity period of enzyme-linked immunosorbent assay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
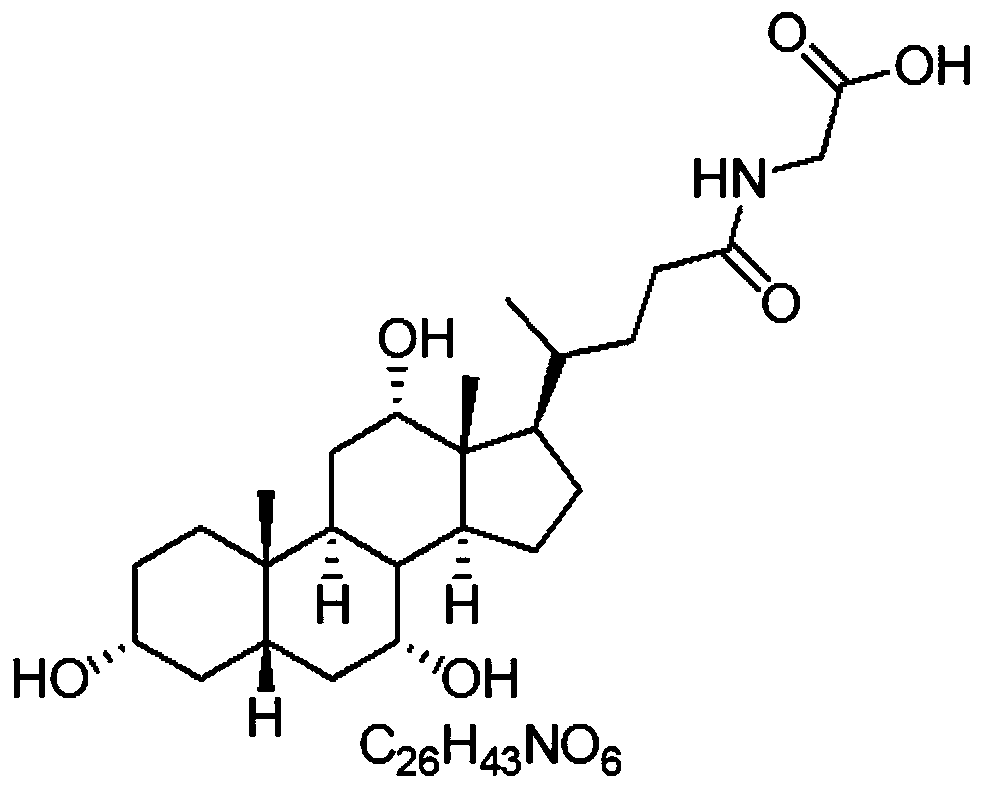
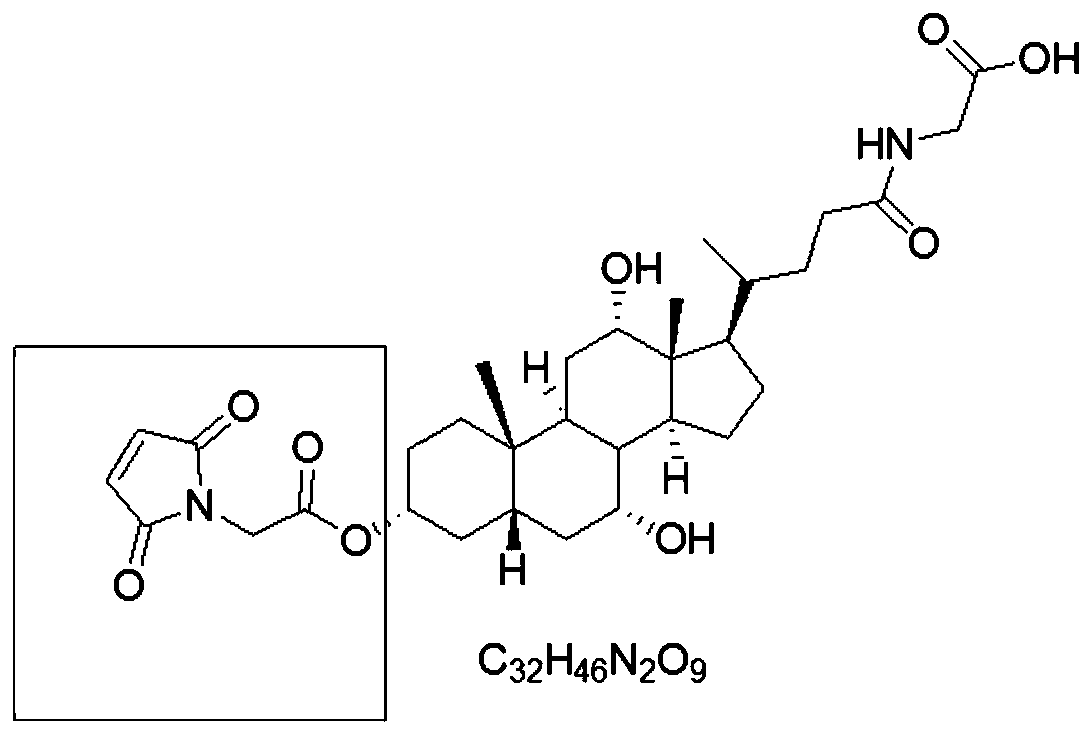
[0072] Embodiment 1. Synthesis of Glycocholic Acid Derivatives
[0073] Add glycocholic acid (1.0eq), maleimidoethylamine (1.0g, 1.0eq) and triethylamine (3.0eq) into a dry and clean 25mL two-necked bottle;
[0074] Then add dimethylformamide (5mL) and stir until completely dissolved, add dichloroethane (1.25eq), and stir at 25°C for 2h;
[0075] HPLC monitoring, until the completion of the reaction;
[0076] Add the above reaction mixture into water (25mL), add ethyl acetate 20mL×3 for extraction;
[0077] Combined organic phases, anhydrous Na 2 SO 4 After drying and concentrating under reduced pressure, the resulting oil was purified by column chromatography to obtain 1.04 g of milky white powdery solid, yield 45%, M+: 602.72.
[0078] The function of this embodiment is to make CG have a group that can bind to enzymes, and the technical effect of this application does not depend on specific hapten derivatives.
Embodiment 2
[0079] Example 2. Coupling of Glycocholic Acid Derivatives to G6PDH Molecules
[0080] According to the G6PDH-glycocholic acid conjugate of the present application, the coupling is carried out in the following manner: the sulfhydryl reactive group (such as maleimide group) on the glycocholic acid derivative molecule co-operates with the thiol group on the G6PDH molecule. price combination.
[0081] 1. Solution preparation:
[0082] Glycocholic acid derivative solution: 10 mg / ml of the glycocholic acid derivative prepared in Example 1 was dissolved in DMF;
[0083] G6PDH solution: 6.7mg / mL G6PDH (mutant or wild type of this application), PB 100mmol, NaCl100mmol, pH=8.0;
[0084] Coupling solution: 100mM PB / K, 100mM EDTA, 150mM NaCl, pH=7.2;
[0085] Desalting solution: 100 mM PB / K, 100 mM EDTA, 150 mM NaCl, pH=7.2.
[0086] 2. Coupling operation: 1.6ml G6PDH solution, 6ml coupling solution and 0.40ml glycocholic acid derivative solution were reacted at room temperature (20 ...
Embodiment 3
[0088] Embodiment 3. Preparation of kit
[0089] Prepare the following kit for detecting glycocholic acid, which comprises:
[0090] Reagent R1, comprising:
[0091] 100mM PB buffer, pH 7.2
[0092] 15mM Glucose 6-phosphate
[0093] 15mM β-nicotinamide adenine dinucleotide
[0094] 0.1mg / L Glycocholic acid antibody
[0095] 200mM NaCl
[0096] 0.5g / L bovine serum albumin
[0097] 0.1g / L Tween20
[0099] Reagent R2, including:
[0100] 100mM PB buffer, pH 7.2
[0101] 0.1mg / L G6PDH-CG conjugate
[0102] 0.5g / L bovine serum albumin
[0103] 0.1g / L Tween 20
[0105] Calibrator: 100mM PB buffer, pH 7.2, and 0, 2.5, 5.0, 10, 20, 40mg / L glycocholic acid (or add as needed);
[0106] Quality control: 100mM PB buffer, pH 7.2, and 1.5, 8.0, 25, 35mg / L glycocholic acid (or add as needed).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com