Preparation method of 3-substituted phenyl-4,5-dihydroisoxazole derivative and application and intermediate thereof
A technology of dihydroisoxazole and derivatives, applied in the direction of organic chemistry and the like, can solve the problems of poor bromination reaction selectivity and unavoidable side reactions, and achieve the effects of safe and controllable production, product quality assurance, and avoidance of by-products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
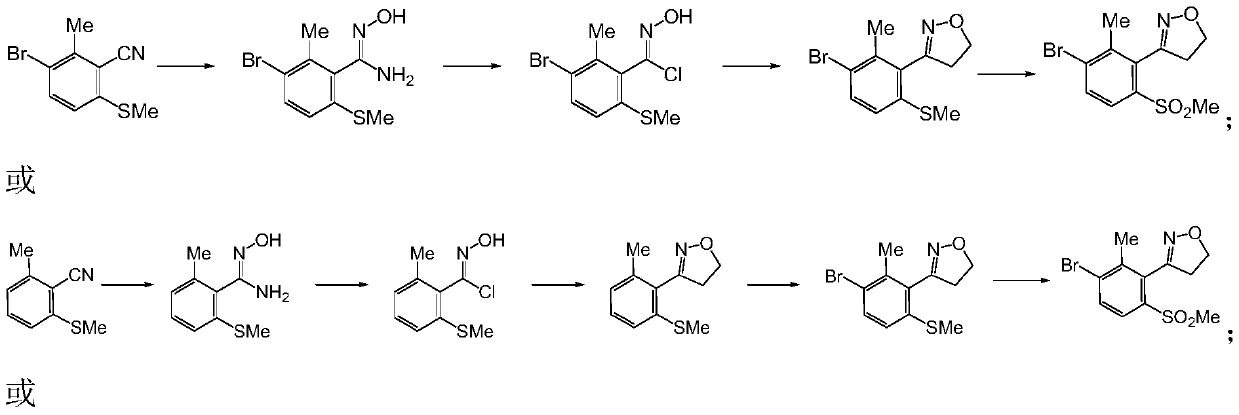
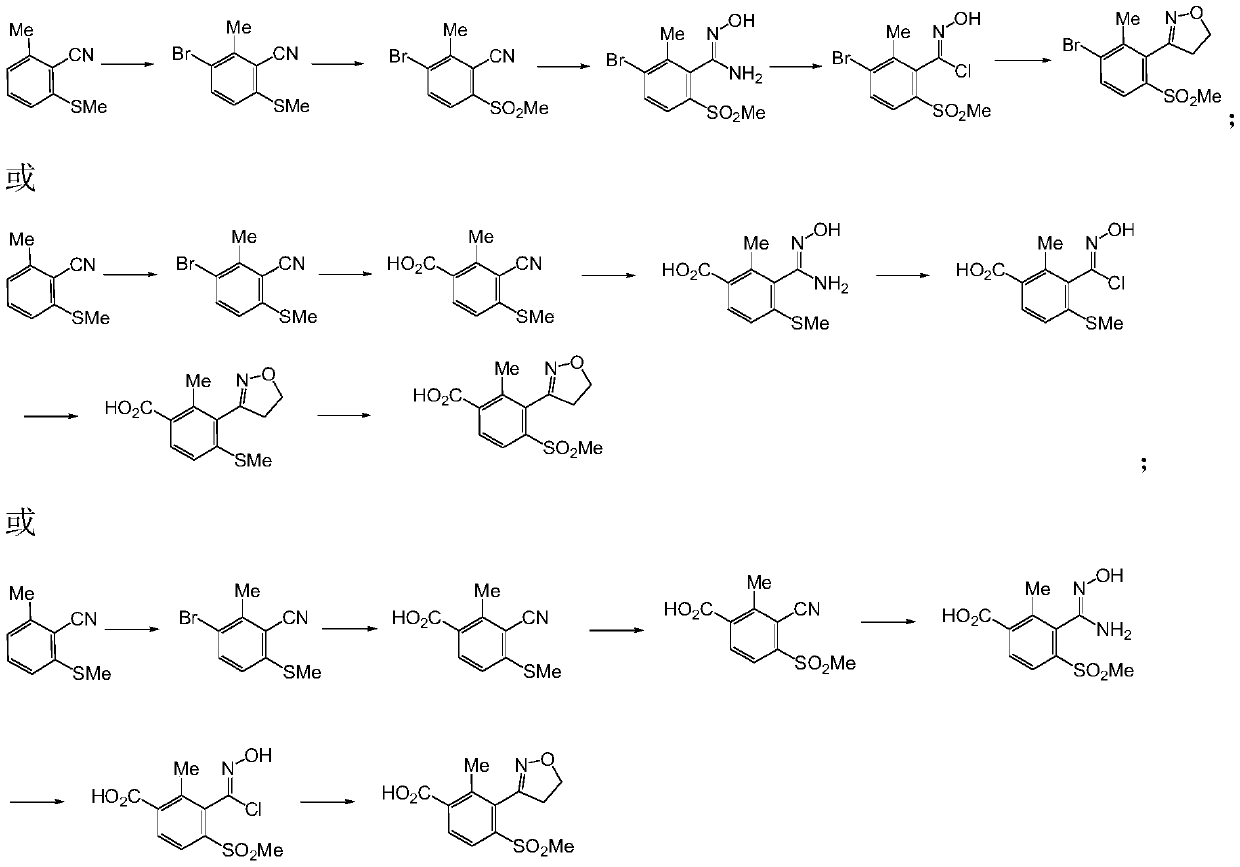
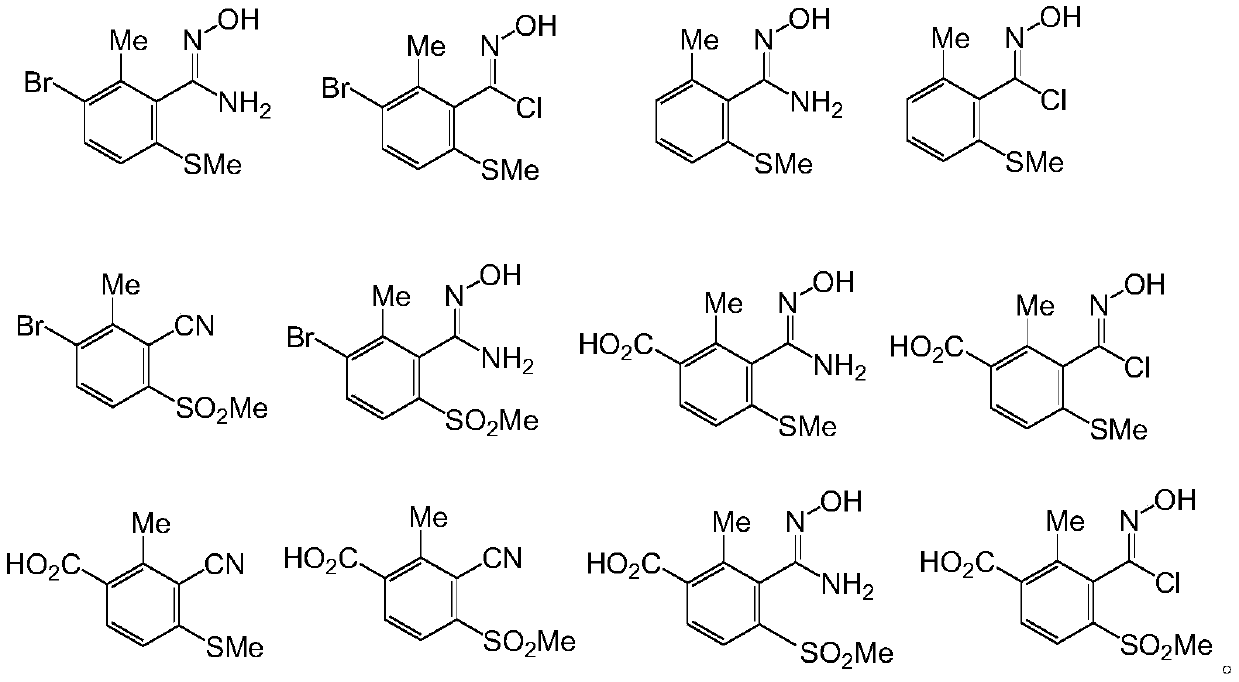
[0071] In the preparation method of the 3-substituted phenyl-4,5-dihydroisoxazole derivatives provided by the present invention, the route and steps are as follows:
[0072]
[0073] (1) The compound of formula V undergoes hydroxylamination reaction to generate the compound of formula VII;
[0074] (2) the compound of formula VII generates the compound of formula VIII through diazotization and halogenation;
[0075] (3) the compound of formula VIII generates the compound of formula IX through dipolar cycloaddition;
[0076] Where: A is selected from H, Cl, Br, I, CO 2 R; R is selected from H, C1-C4 alkyl; X is selected from Cl, Br or I; n is selected from 0, 1 or 2.
[0077] Concrete reaction condition is as follows:
[0078] step 1):
[0079]
[0080] n=0,1,2A=-Cl,-Br,-I,-CO 2 H,-CO 2 Me,-CO 2 Et et al.
[0081] The reaction is carried out under the following conditions: the solvent used is a protic solvent such as: water, methanol, ethanol, propanol, isopropano...
Embodiment 1
[0103] Synthesis of 2-methyl-6-methylthiobenzonitrile
[0104]
[0105] Add 2-methyl-6-nitrobenzonitrile (2.49g, 15.36mmol), toluene 40mL, 20% sodium methyl mercaptide solution (5.38g, 15.37mmol) successively to a one-necked bottle equipped with a magnetic stirring bar, TBAB 0.30g. After stirring at room temperature for 4 hours, it was detected by thin layer chromatography that the reaction was complete. Add 50 mL of distilled water, shake, let stand, and separate the toluene phase. Wash with saturated aqueous sodium chloride (10 mL×3), and dry over anhydrous sodium sulfate. After precipitation under reduced pressure, the crude product was purified by column chromatography to obtain white crystals (2.09 g, 83.4%). ESI-MS(m / z):164[M+H] + , 186[M+Na] + , 202[M+K] + .
Embodiment 2
[0107] Synthesis of 3-bromo-2-methyl-6-methylthiobenzonitrile
[0108]
[0109] Add 2-methyl-6-methylthiobenzonitrile (0.41 g, 2.51 mmol), iron tribromide (0.95 g, 3.21 mmol) and 10 mL of dichloromethane into a 25 mL two-necked flask equipped with a thermometer to dissolve. After cooling down to -5-0°C, magnetic stirring was started, and liquid bromine (0.95 g, 5.93 mmol) was added dropwise. After 1 hour, it was detected by thin-layer chromatography that the reaction was complete. Add 10 mL of distilled water to quench, extract with dichloromethane (10 mL×3), wash the organic phase with 3% aqueous sodium hydroxide solution (10 mL×3), and then wash with distilled water until neutral. The organic phase was dried over anhydrous sodium sulfate, desolvated under reduced pressure and purified by column chromatography to obtain white crystals (0.55 g, 90.4%). ESI-MS(m / z):242[M+H] + , 264[M+Na] + , 280[M+K] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com