Dominant epitope peptide of anti-Mrgprx2 antibody and application thereof
A dominant antigen and epitope peptide technology, applied in the field of biomedicine, can solve the problem that there are no relevant clinical detection kits and drugs for MRGPRX2 diagnosis and treatment, and achieve strong immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
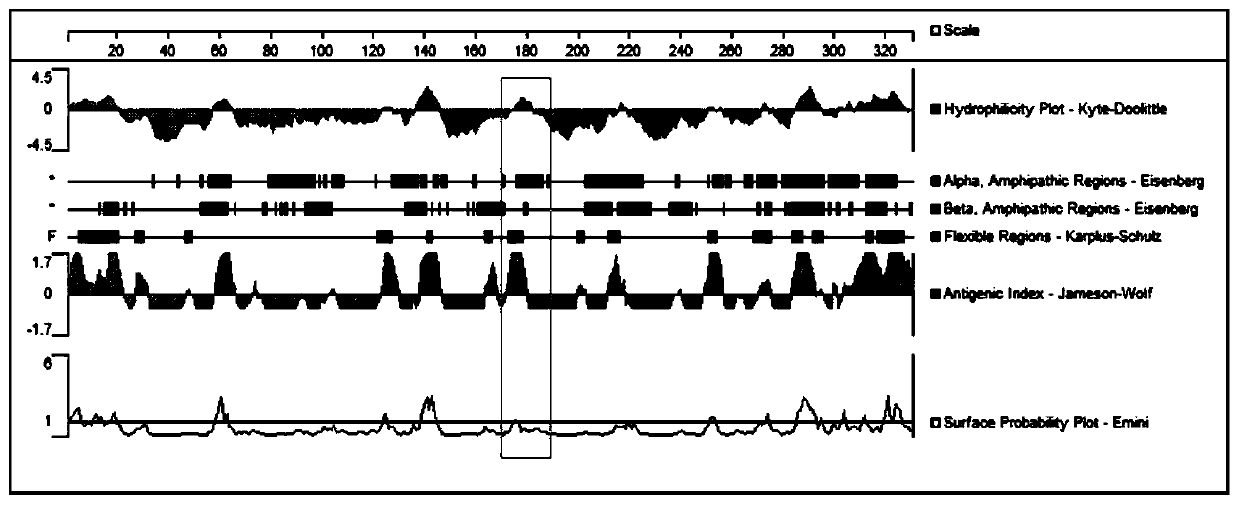
[0027] Example 1 Synthesis of the Antigen Peptide of the Class 1 Anaphylaxis Specific Receptor MRGPRX2 Protein
[0028] DNAstar analysis software was used to predict and analyze the epitopes of human MRGPRX2 amino acid sequence, such as protein hydrophilicity, sequence flexibility, protein surface accessibility, and protein antigenic index, and finally determined the 166th-184th position as the target, amino acid The sequence is KFCGFLFSDGDSGWCQTFD (as shown in SEQ ID NO: 1). The manual solid-phase Fmoc method is used to synthesize from the C-terminal to the N-terminal direction to obtain the crude product of the target polypeptide. Purify the target polypeptide by using reversed-phase high-performance liquid chromatography (HPLC) method to separate different polypeptide molecules according to the difference in hydrophobicity. After freeze-drying the solvent, the pure polypeptide in a fluffy state was obtained. Its chemical structure was characterized by MALDI-TOF mass spectr...
Embodiment 2
[0030] Embodiment 2 Preparation of anti-polypeptide mouse monoclonal antibody
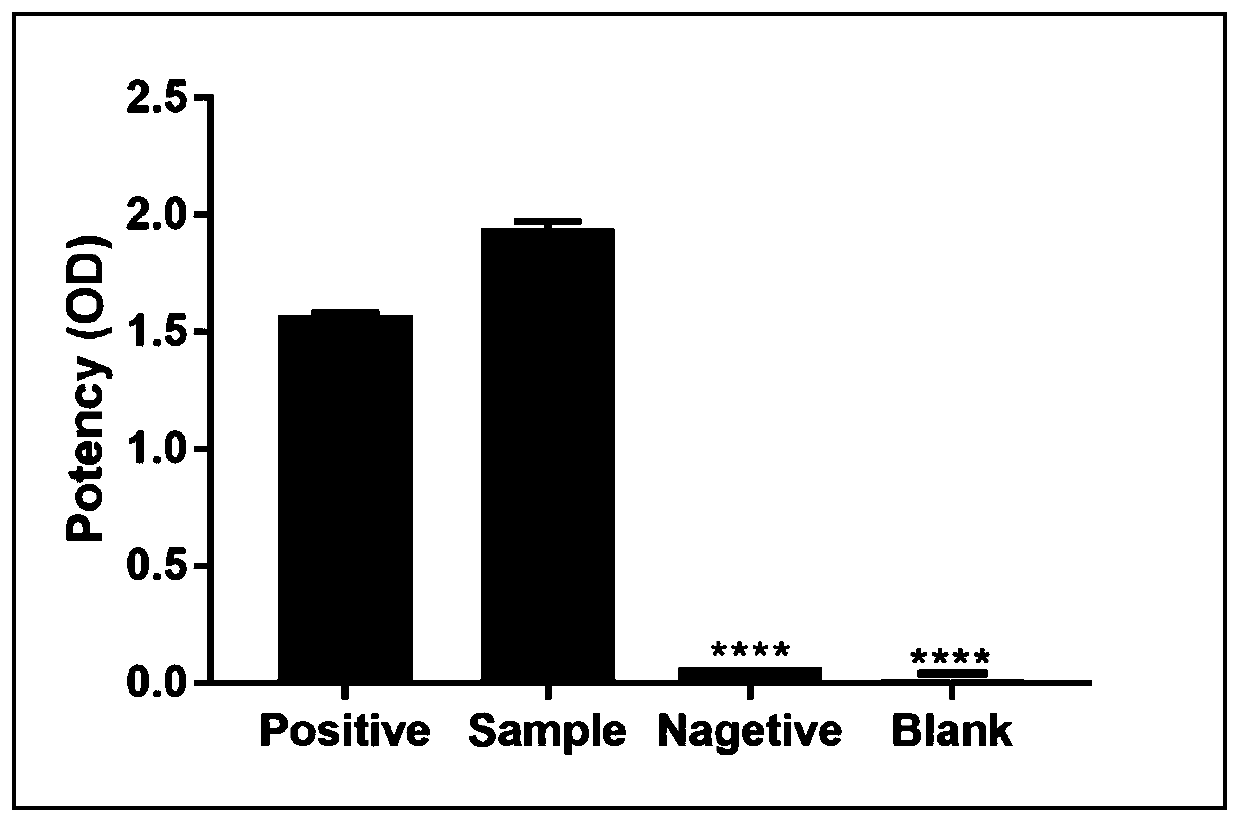
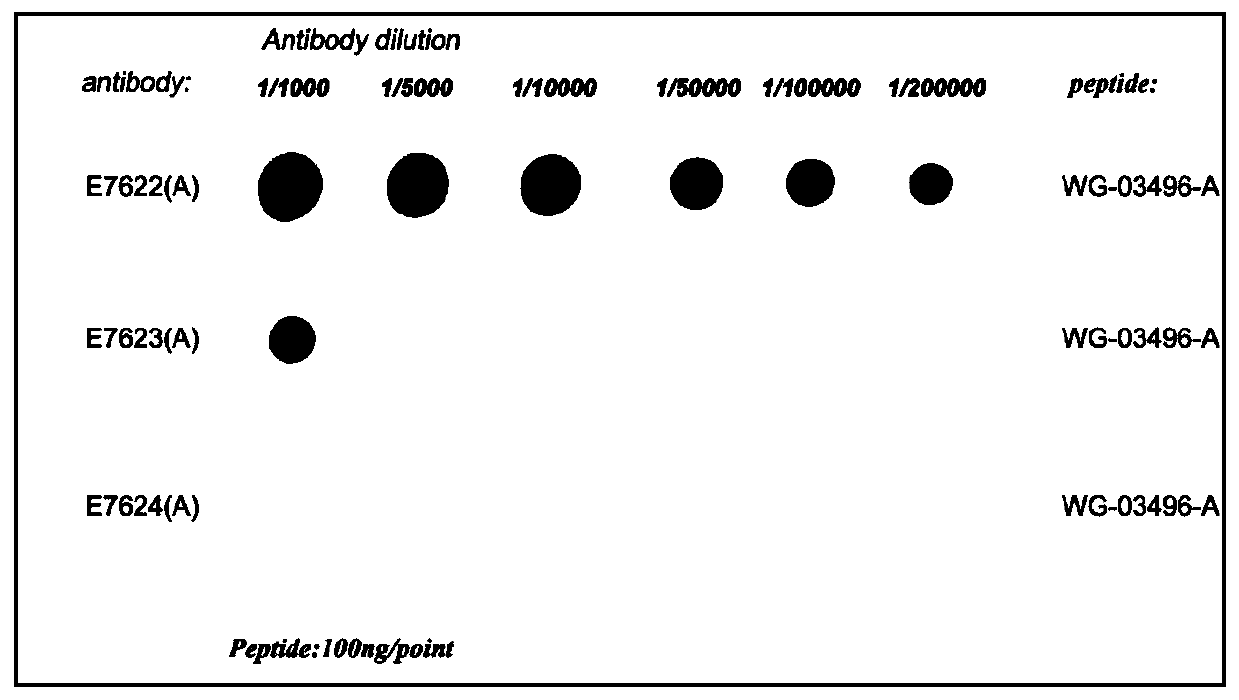
[0031] Prepare the conjugated KLH-polypeptide into an emulsified injection for immunization, and inject subcutaneously in points, spray alcohol on the back midline of the mouse, avoid the parts with immune swelling, and inject one injection into four times, respectively. 4 different points. After five immunizations, blood was collected from the tail vein of the mice to detect the titer of antiserum by indirect ELISA. The ELISA antiserum titer reached 1:50000, indicating that the immunity was qualified. Select the mouse with the highest titer for fusion screening, and then carry out subcloning, and screen pure monoclonal cell lines according to the results of subcloning. The supernatant of the monoclonal cell line was injected into mice to prepare ascites, and the ascites was taken for protein G affinity purification to obtain purified monoclonal antibodies.
Embodiment 3
[0032] Example 3 Identification of Antibody: Using Indirect ELISA to Detect Monoclonal Antibody Titer
[0033] 1) Microtiter plate preparation: After assembling the microtiter plate, prepare for antigen coating.
[0034] 2) Antigen dilution and coating: Add the prepared antigen working solution into the concave sample tank, take 100 μL of the antigen working solution with a 300 μL range 8-hole pipette and add it to the bottom of the microplate well. After adding the sample, cover it Aluminum foil paper, incubate in an oven at 37°C for 2h or overnight at 4°C.
[0035]3) Plate washing: Take out the ELISA microplate plate coated with antigen, turn it upside down to remove the liquid in the well, and then place it on a clean absorbent towel to drain; add 200ml TBST to each well with a drain gun until it is full but not If it overflows, after adding the whole plate and let it stand for 3 minutes, shake out the TBST for 3 times, then place it on a clean absorbent towel to drain, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com