Preparation method of pseudomonas protegens transcription factor LexA polyclonal antibody
A Pseudomonas mutans and polyclonal antibody technology, applied in the biological field, achieves the effect of simple method and strong immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for preparing a polyclonal antibody against Pseudomonas mutans transcription factor LexA in this example, wherein the specific implementation steps for constructing a recombinant plasmid are as follows: artificially synthesize the LexA gene according to the sequence of the Pseudomonas mutans LexA gene obtained by previous genome sequencing and insert it into the pET-32 expression vector, wherein the gene nucleic acid sequence is inserted as figure 1 Shown in SEQ ID NO: 1; the pET-32 vector is ampicillin-resistant and contains Trx tags and 6xHis tags. The Trx thioredoxin tag is beneficial to enhance the soluble expression of the protein, the molecular weight is about 20kDa, and it is located at the N-terminal of the target gene in the pET-32 vector. Transform the constructed recombinant plasmid into Escherichia coli DH5α, use an LB plate containing 100 μg / ml ampicillin, incubate at 37°C for 12 hours, pick a single colony and shake it back, extract the plasmid an...
Embodiment 2
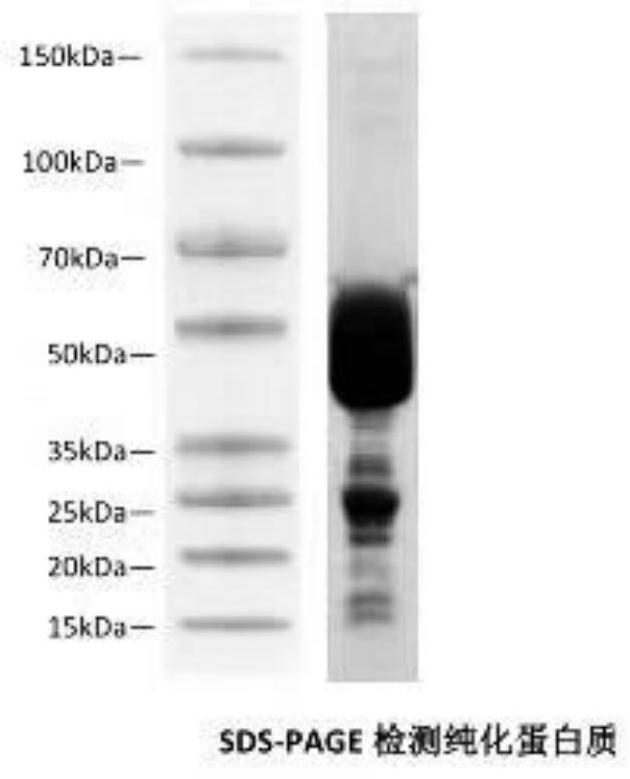
[0028] A method for preparing a polyclonal antibody against Pseudomonas mutans transcription factor LexA in this example, wherein the small sample expression specific implementation steps are as follows: transform the recombinant plasmid constructed as described in Example 1 into BL21 DE3 competent cells, and inoculate resistant LB Plate medium, grow for 24 hours; select 6 single clones from the transformed plate, inoculate 3ml of resistant liquid medium respectively; culture at 37°C, 220RPM to OD600nm0.5-0.6, add 0.5mM IPTG at 20°C to induce expression for 3.5 hours; centrifuge The cells were collected, sonicated, and the expression was detected by SDS-PAGE. The detection results showed that the protein was expressed in both the supernatant and the inclusion body, and the soluble expression and purification could be continued.
Embodiment 3
[0030]A method for preparing a polyclonal antibody against Pseudomonas mutans transcription factor LexA in this example, wherein the specific implementation steps for large-scale expression are as follows: select a small-scale well-expressed strain as described in Example 2 and inoculate 60 μl to 200 ml of resistant medium, Cultivate at 37°C for 24 hours; add fresh resistant medium to 800ml, incubate for 1-2 hours, to OD600nm0.5-0.6; add 200μl of 1M IPTG (28°C or 37°C) to induce expression for 3.5 hours; collect by centrifugation at 4°C Bacteria (66 rpm × 15min), precipitate and discard the supernatant, add 30ml PBST to suspend the bacteria, add a final concentration of 1mM PMSF, and ultrasonically break at 200W for 6min in an ice bath; incubate at 4°C for 1 hour on a shaker; 133rpm Centrifuge at high speed for 15 minutes, take the supernatant, add 400μl nickel column to combine at 4°C for 24 hours; collect the nickel column (33 rpm x 5min), wash the beads with 20mM imidazole w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com