Preparation method and application for glycosylated naringenin
A technology of naringenin and glycosylation, applied in the field of bioengineering, can solve problems such as low bioavailability of naringenin, complex extraction, separation process, research affecting pharmacological activity, etc., to achieve improved bioavailability and broad development Foreground, effect of solubility improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of preparation method of glycosylated naringenin, its concrete steps comprise:
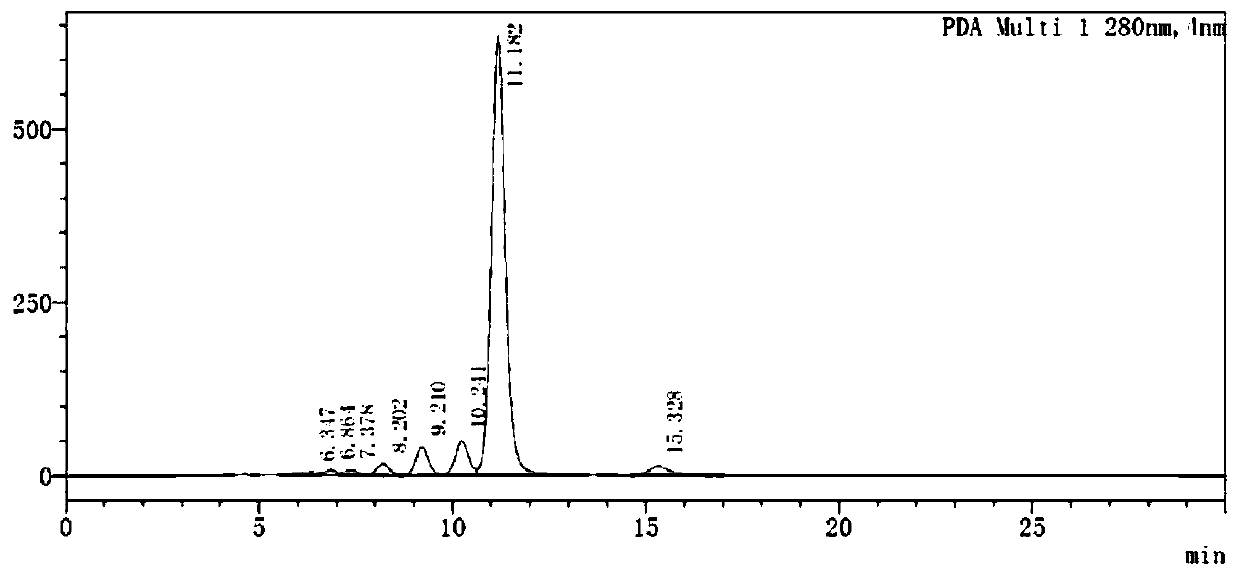
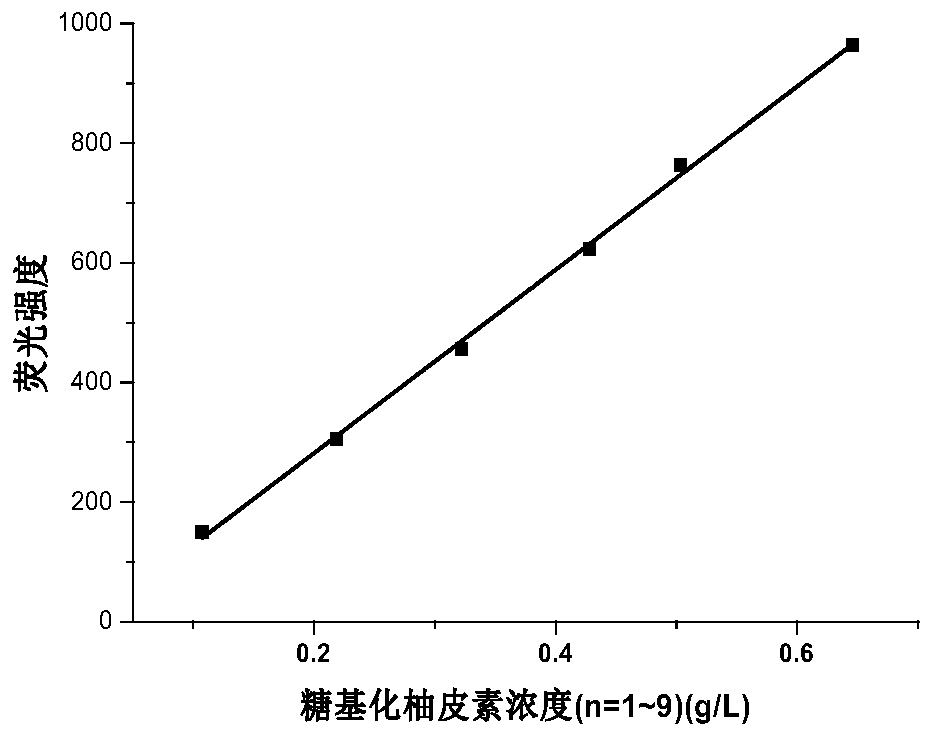
[0034] Using naringin as raw material, the reaction buffer is disodium hydrogen phosphate-sodium citrate buffer with a mass fraction of 0.9%, the pH of the reaction buffer is 5.7, the ratio of raw material to reaction buffer is 1:22, and the reaction temperature is 60 ℃, the reaction time is controlled for 15 hours, then rhamnosidase:glucosyltransferase=10:90 in the reaction system, the mass fraction of the total enzyme is 15% of the input naringin. Add 100g of 90% naringin, the glycosyl donor is glucose, and the concentration is 6g / L. In this reaction system, 155.9g (n=1-9) of polyglucose naringenin is obtained, with a yield of 96.5%. The results are shown in figure 1 , due to the difference in the number of sugar groups linked by polyglucose naringenin, a reduced peak will appear in the peak time of the HPLC graph.
Embodiment 2
[0036] A kind of preparation method of glycosylated naringenin, its concrete steps comprise:
[0037] Using naringenin as raw material, the reaction buffer is disodium hydrogen phosphate-sodium citrate buffer with a mass fraction of 0.9%, the pH of the reaction buffer is 5.7, the reaction temperature is 46°C, and the ratio of raw materials to reaction buffer is 1: 10. The reaction time is controlled for 15 hours; then in the reaction system, rhamnosidase:glucosyltransferase=90:10, the mass fraction of the total enzyme is 10% of the input naringenin; input 100g 90% naringenin, glycosyl The donor was glucose at a concentration of 1 g / L. In this reaction system, 75.1 g of monoglucoside naringenin was obtained with a yield of 93.8%.
Embodiment 3
[0039] A kind of preparation method of glycosylated naringenin, its concrete steps comprise:
[0040] Using naringin as raw material, the reaction buffer is disodium hydrogen phosphate-sodium citrate buffer with a mass fraction of 0.9%, the pH of the reaction buffer is 5.7, the ratio of raw material to reaction buffer is 1:50, and the reaction temperature is 55 ℃, the reaction time is controlled for 8 hours; then in the reaction system, rhamnosidase:glucosyltransferase=10:90, the mass fraction of the total enzyme is 25% of the input naringin; input 100g 90% naringin, glycosyl The donor was glucose at a concentration of 10 g / L. In this reaction system, 130.6 g (n=1-9) of polyglycosylated naringenin was obtained, and no naringenin was detected by HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com