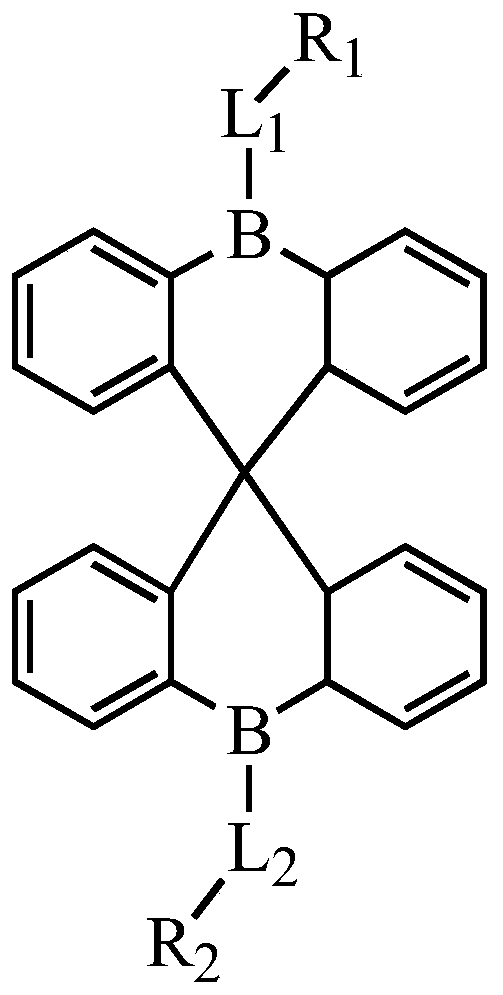
Compounds, display panel and display device
A compound and connection position technology, which is applied in the field of organic electroluminescence materials, can solve the problems of large free volume, decrease in luminous efficiency, breakage, etc., and achieve the effects of improving fluorescence quantum efficiency, increasing luminous efficiency, and reducing device voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Synthesis of Compound M1
[0096]
[0097] Add 12.48g (80mmol) of Compound A into a 200mL three-necked flask, add 0.5g of anhydrous aluminum trichloride to Compound A and mix well, and add 3.08g of dry Compound B in portions while stirring at 30-40°C (20mmol), and then continue to stir until the reaction is no longer exothermic, the steam is kept at 70-80°C, and the mixture is refluxed until the hydrogen chloride escapes gently. Add 20mL of 6mol / L hydrochloric acid into benzene, mix evenly, and add the above mixed solution in portions under rapid stirring to carry out hydrolysis reaction, and control the hydrolysis temperature below 40°C. Separate the benzene layer, dilute the water layer with ice water, and extract several times with benzene, combine the extracts, dry with anhydrous calcium chloride, decolorize with activated carbon, filter and cool to crystallize. The crystals were dissolved in a mixed solvent of benzene and petroleum ether with a little chloroace...
Embodiment 2
[0106] Synthesis of compound M6
[0107]
[0108] Add 12.48g (80mmol) of Compound A into a 200mL three-necked flask, add 0.5g of anhydrous aluminum trichloride to Compound A and mix well, and add 3.08g of dry Compound B in portions while stirring at 30-40°C (20mmol), and then continue to stir until the reaction is no longer exothermic, the steam is kept at 70-80°C, and the mixture is refluxed until the hydrogen chloride escapes gently. Add 20mL of 6mol / L hydrochloric acid into benzene, mix evenly, and add the above mixed solution in portions under rapid stirring to carry out hydrolysis reaction, and control the hydrolysis temperature below 40°C. Separate the benzene layer, dilute the water layer with ice water, and extract several times with benzene, combine the extracts, dry with anhydrous calcium chloride, decolorize with activated carbon, filter and cool to crystallize. The crystals were dissolved in a mixed solvent of benzene and petroleum ether with a little chloroace...
Embodiment 3
[0120] Synthesis of Compound M7
[0121]
[0122] Add 12.48g (80mmol) of Compound A into a 200mL three-necked flask, add 0.5g of anhydrous aluminum trichloride to Compound A and mix well, and add 3.08g of dry Compound B in portions while stirring at 30-40°C (20mmol), and then continue to stir until the reaction is no longer exothermic, the steam is kept at 70-80°C, and the mixture is refluxed until the hydrogen chloride escapes gently. Add 20 mL of 6 mol / L hydrochloric acid to benzene, mix well, and add the above mixed solution in portions under rapid stirring to carry out hydrolysis reaction, and control the hydrolysis temperature below 40°C. Separate the benzene layer, dilute the water layer with ice water, and extract several times with benzene, combine the extracts, dry with anhydrous calcium chloride, decolorize with activated carbon, filter and cool to crystallize. The crystals were dissolved in a mixed solvent of benzene and petroleum ether with a little chloroacety...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com