Baicalin liposome ointment and preparation method thereof
A technology of baicalin and baicalin, applied in the field of baicalin liposome ointment and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of Baicalin Liposomes
[0031] Weigh 18 mg of baicalin powder into a container, add 6 mL of pH 7.0 phosphate buffer solution, stir to dissolve completely, and obtain a 3 mg / mL baicalin solution. Separately weigh 200 mg of soybean lecithin, 2 mg of vitamin E, and 34 mg of cholesterol into a beaker, add 6 mL of ether and 12 mL of chloroform to mix and dissolve, stir well, and slowly pour into the prepared baicalin solution. Sonicate in a water bath for 8 minutes to obtain a yellow upper layer and a milky white lower layer solution. Rotate to a colloidal state at 40°C. Add 1.5mL Tween 80 and 5mL pH7.0 phosphate buffer solution, continue to rotate and mix until a pale yellow solution is formed. Baicalin liposomal suspension.
Embodiment 2
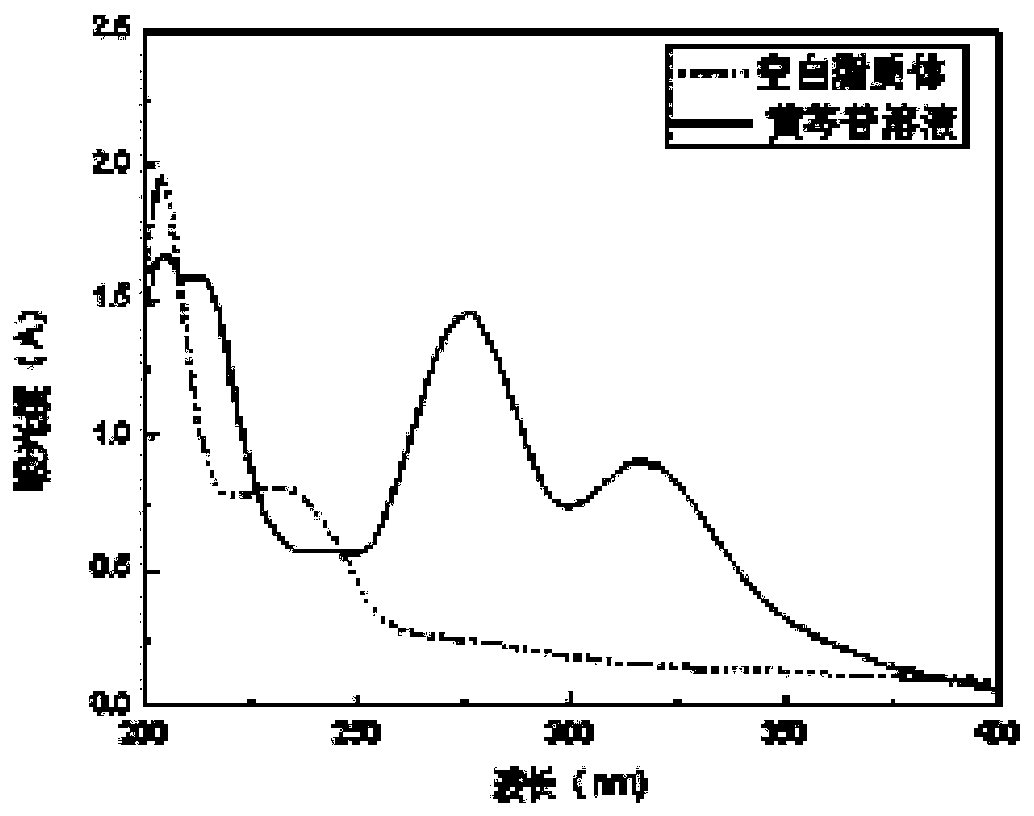
[0033] Selection of Baicalin Detection Wavelength
[0034] Take an appropriate amount of baicalin solution and the prepared blank liposomes, and dilute them in a 10 mL volumetric flask with normal saline, respectively. Full-wavelength scanning was performed using UV spectrophotometry, ranging from 200-400 nm. Depend on figure 1 It can be seen that the baicalin solution has a maximum absorption wavelength at 214nm and 276nm, and the blank liposome has a greater impact on it at 214nm, while the blank liposome does not interfere with it at 276nm, so it is advisable to select the detection wavelength at 276nm.
Embodiment 3
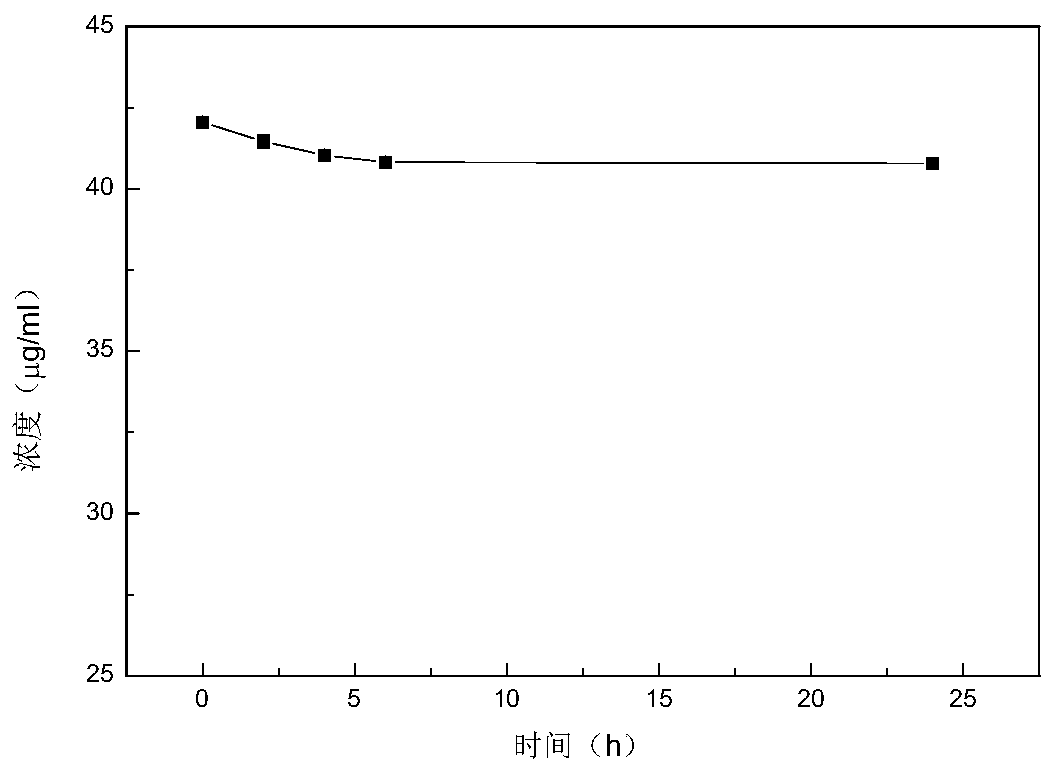
[0036] Determination of Encapsulation Efficiency of Baicalin Liposome
[0037] Take 1 mL of baicalin liposome suspension and put it into an ultrafiltration centrifuge tube, centrifuge in a refrigerated centrifuge for 30 min, and adjust the speed to 7000 r / min. Take all the filtrate at the bottom of the ultrafiltration centrifuge tube and dilute to constant volume, measure its absorbance, combine the standard curve to calculate the free baicalin concentration as c 2 . Take another 1mL baicalin liposome suspension of the same batch, dilute to the same multiple, measure its absorbance with UV, and convert the total concentration of baicalin into c 1 . The encapsulation rate calculation formula is:
[0038] Several batches of baicalin liposomes were prepared, and the encapsulation efficiency was (85.24±2.89)% measured under the same conditions, and the RSD was 3.39%. The specific results are shown in Table 1.
[0039] Table 1 baicalin liposome encapsulation efficiency assay ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com