Coumarin-benzothiazolyl hydrazone compound, preparation method and application thereof
A technology of benzothiazolyl hydrazone and coumarin, which is applied in chemical instruments and methods, organic chemistry, and analysis through chemical reactions of materials, can solve the problems of expensive and cumbersome pretreatment, and achieve strong anti-interference The effect of ability, good application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the synthesis of compound CTS
[0035] Weigh 2.45g (10mmoL) 3-formyl-7-N, N-diethylaminocoumarin and 1.65g (10mmoL) 2-hydrazinobenzothiazole respectively and dissolve them in 10mL absolute ethanol, stir to make Fully dissolve, add 0.5mL acetic acid as a catalyst, heat up to 75°C and reflux for 6h, filter the reaction solution after cooling, wash, dry, recrystallize from absolute ethanol, and obtain 2.50g of a yellow solid after drying, which is the target product, with a yield of 70.4 %.
[0036] FT-IR (KBr, cm -1 ): 3434.32 (-N-H), 2931, 2872 (-CH 3 ,-CH2 ), 1727(-C=O), 1624(-C=N-), 1602(-Ar), 1349(-C-H), 1131(-C-N), 638(-N-H).
[0037] 1 H NMR (400MHz, DMSO-d 6 ): δ(ppm)8.24(s,1H),8.14(s,1H),7.78(d,1H,J=8.0Hz),7.64(d,1H,J=8.0Hz),7.45(d,1H) ,7.30(t,1H),7.13(t,1H),6.77(d,1H,),6.59(s,1H),3.42(m,4H),1.15(t,6H).MS-ESI: m / z[M+H] + ,393.26 for C 21 h 20 N 4 o 2 S.
Embodiment 2
[0038] Embodiment 2: the ultraviolet-visible absorption spectrometry of compound CTS
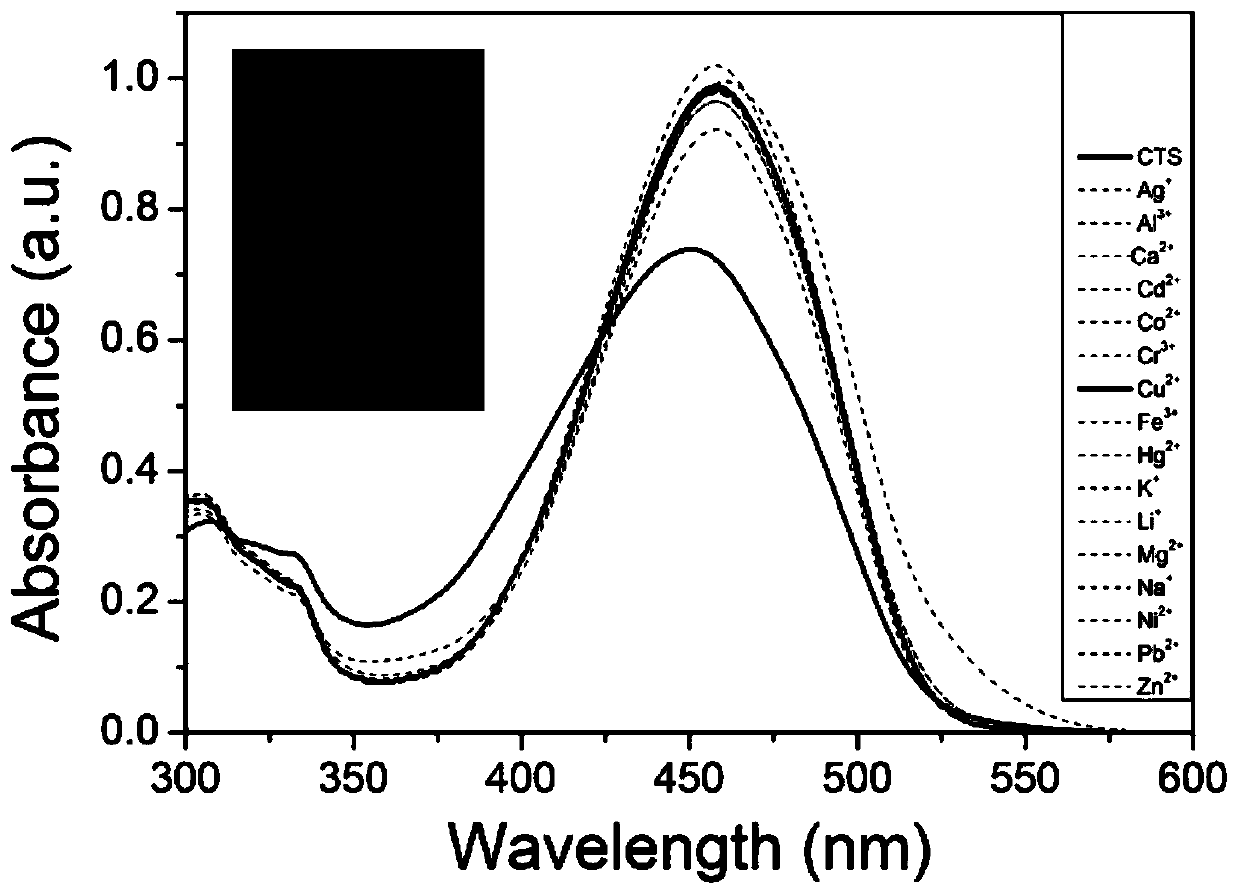
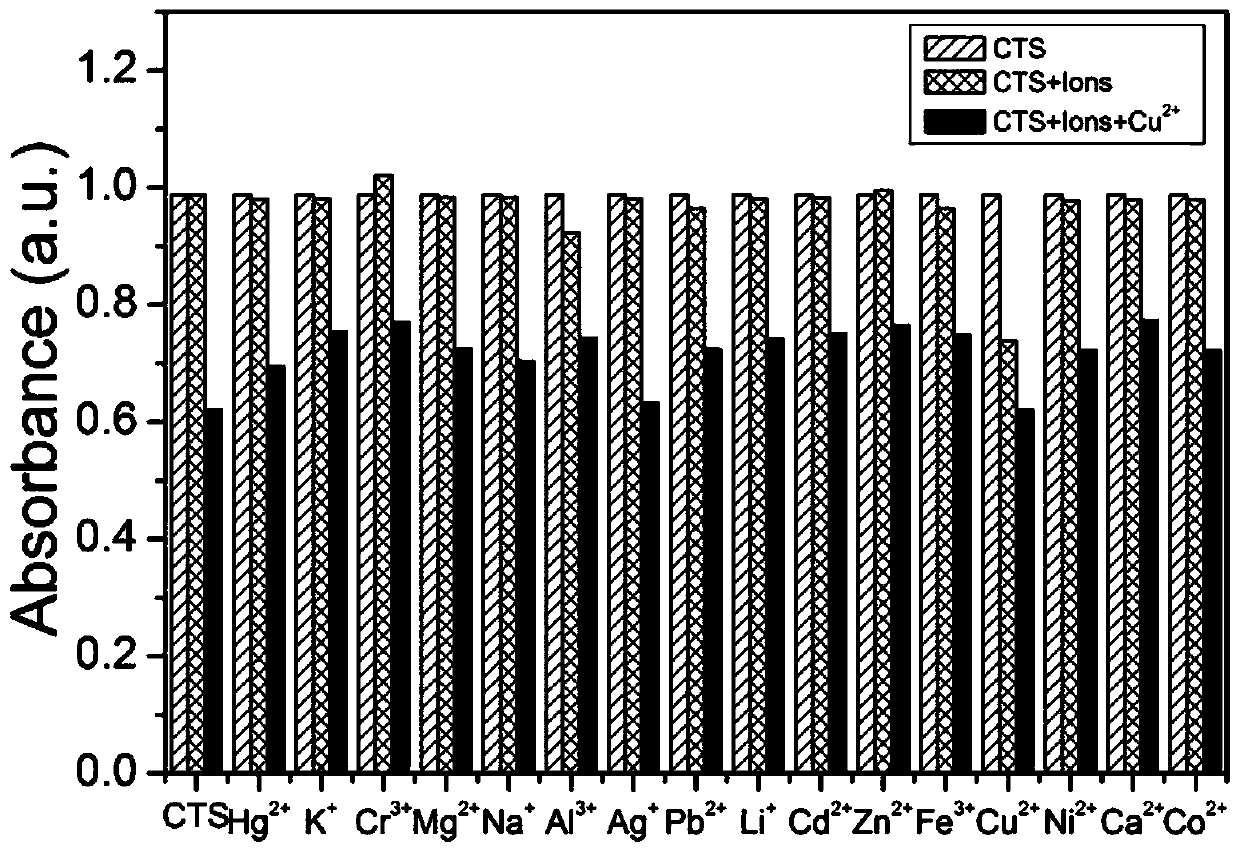
[0039] Accurately weigh 3.9 mg of compound CTS, dissolve and prepare a concentration of 1.0×10 -2 mol / L acetonitrile stock solution; dilute the stock solution to a concentration of 1.0×10 with a mixture of acetonitrile and water at a volume ratio of 7:3 -5 mol / L of the solution to be tested. Take 3mL concentration as 1.0×10 -5 mol / L of the solution to be tested in a quartz cuvette (the thickness of the quartz cuvette is 1cm), and then add 3 μL of the solution at a concentration of 1.0×10 -2 mol / L of various metal ions (Na + , K + , Ag + , Ca 2+ , Mg 2+ , Pb 2+ , Li + , Al 3 + , Zn 2+ , Cd 2+ , Cr 3+ , Cu 2+ , Ni 2+ ,Co 2+ , Fe 3+ , Hg 2+ ) aqueous solution, shake well, and measure the UV-Vis absorption spectrum of the solution after 1 minute (see accompanying drawing figure 1 ). Before adding metal ions, the UV-Vis absorption spectrum of compound CTS showed an obvious ab...
Embodiment 3
[0040] Embodiment 3: the determination of the ultraviolet-visible absorption spectrum titration experiment and detection limit of compound CTS
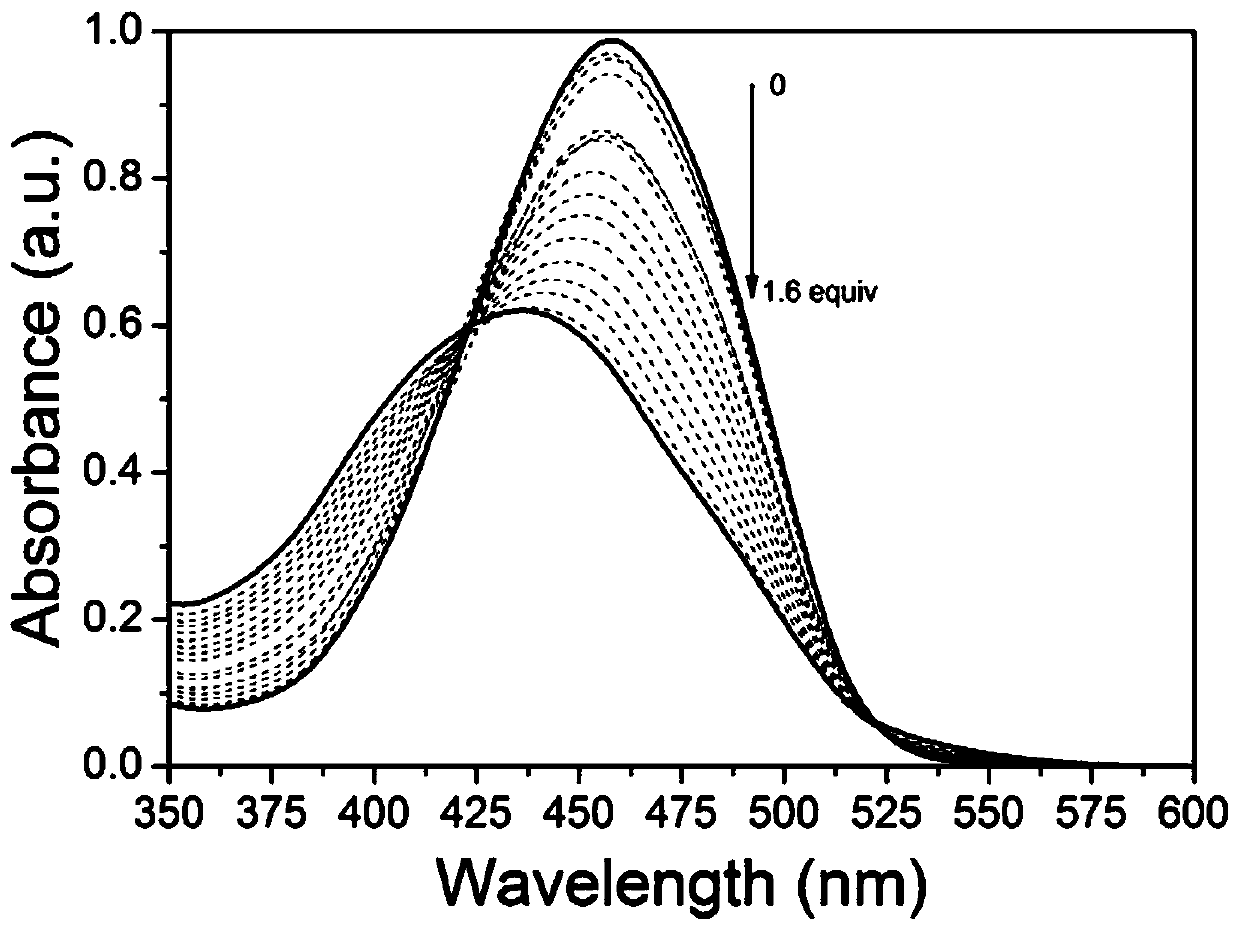
[0041] Take 3mL concentration as 1.0×10 -5 mol / L of the solution to be tested is placed in a quartz cuvette, and the concentration is 1.0×10 -3 mol / L Cu 2+ Ionic aqueous solution, measure the ultraviolet-visible absorption spectrum of solution after shaking up (as accompanying drawing figure 2 shown). With Cu 2+ With the addition of ions, the absorbance of compound CTS at 459nm gradually weakens and blue-shifts to 450nm, and an obvious equivalence point appears at 450nm, indicating that compound CTS can with Cu 2+ ions form stable complexes, Cu 2+ ions at 1.0 x 10 -6 ~1.6×10 - 5 In the range of mol / L, Cu 2+ There is a good linear relationship between the ion concentration and the change of absorbance at 450nm (R 2 =0.98), through calculation, the compound CTS effect on Cu 2+ The detection limit of ions is 2.40×10 -6 mol / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com