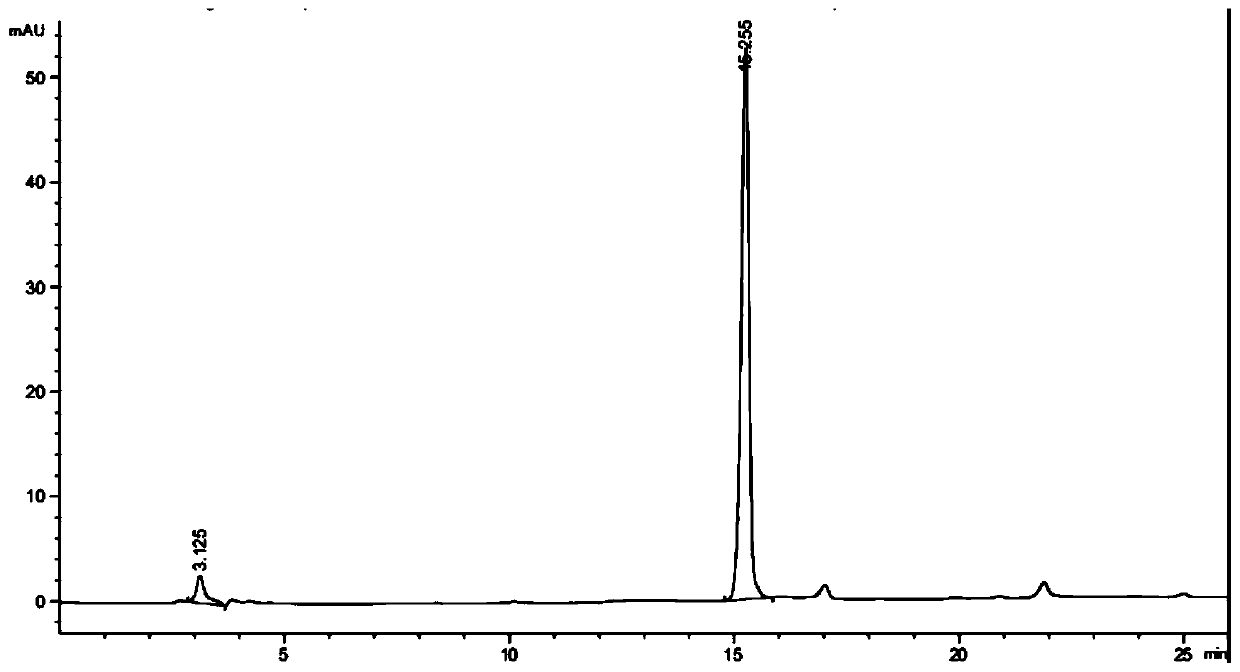
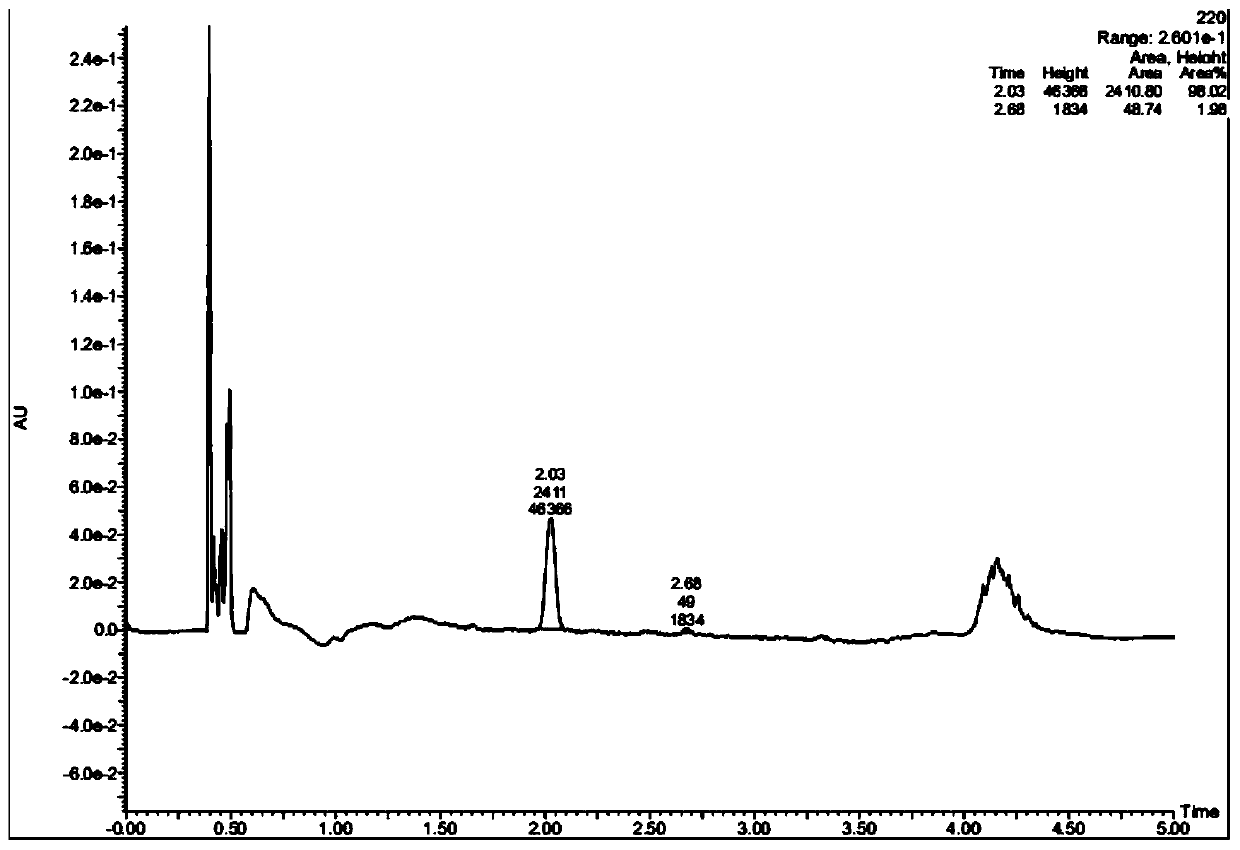
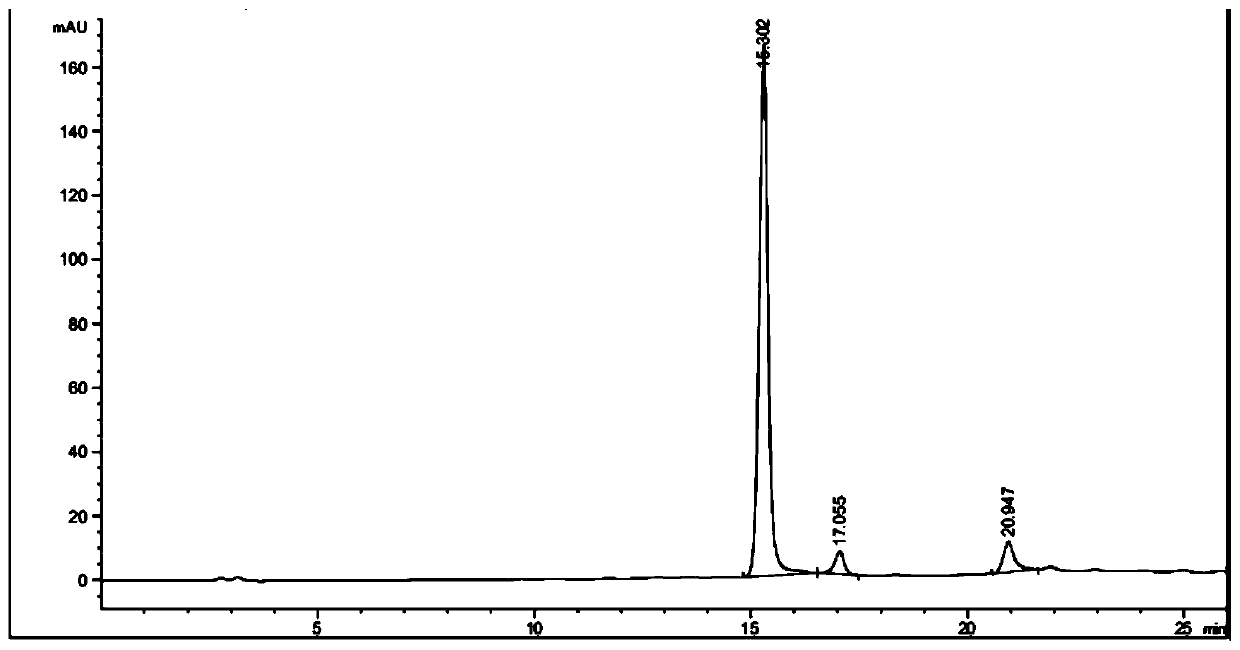
Ketoreductase mutant and application thereof in preparing duloxetine chiral alcohol intermediate and analogues thereof
A technology of reducing enzymes and mutants, which is applied in the fields of biological enzyme engineering and microbial applications, can solve the problems of unreported research on enzymes and their mutants, and achieve the effects of large-scale industrial application prospects, mild reaction conditions, and stable coenzyme cycle system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] The construction of embodiment 1 prokaryotic expression system
[0080] The ketoreductase Rr Kred gene fragment whose nucleotide sequence is shown as SEQ ID NO: 2 was synthesized by Nanjing GenScript Biotechnology Co., Ltd., and recombined into pET21a vector. The positive recombinant plasmid Rr Kred-pET21a (+) was transformed into the expression host strain BL21 (DE3) (purchased from Tiangen Biochemical Technology (Beijing) Co., Ltd.), and the prokaryotic expression strain Rr Kred-pET21a (+) / BL21 (DE3) was obtained. Primary strains used as subsequent catalytic reactions.
[0081] Glucose dehydrogenase (BsGDH) (LOC111893255) and alcohol dehydrogenase (TbADH) (LOC101068320) genes for coenzyme regeneration were synthesized by Nanjing GenScript Co., Ltd. ) plasmids were constructed and transformed into BL21(DE3) to obtain BsGDH-pET21a(+) / BL21(DE3) and TbADH-pET21a(+) / BL21(DE3) expression strains respectively.
Embodiment 2
[0082] Fermentation preparation of embodiment 2 enzyme
[0083] The expression strains Rr Kred-pET21a(+) / BL21(DE3), BsGDH-pET21a(+) / BL21(DE3) and TbADH-pET21a(+) / BL21(DE3) constructed above were added with a final concentration of 100ug / mL Ampicillin in 5mL LB liquid medium [10g / L tryptone (OXIOD), 5g / L yeast powder (OXIOD), 10g / L sodium chloride (Sinopharm Reagent)] was shaken at 37°C and 200rpm overnight, then press 1% (V / V) ratio was inoculated in 500 mL LB liquid medium containing ampicillin with a final concentration of 100 ug / mL, and cultured with shaking at 37° C. and 200 rpm. When the OD600 was between 0.8-1.0, the inducer IPTG (isopropyl-β-D-thiogalactopyranoside, IPTG) was added at a final concentration of 0.1 mM, and induced overnight at 25°C. The bacteria were collected by centrifugation at 8000rpm, then suspended in 50mM pH7.0 sodium phosphate buffer, ultrasonically disrupted (200W, 3s / 5s, 10min), centrifuged at 12000rpm at 4°C for 20min, and the supernatant was ...
Embodiment 3
[0084] The catalytic activity detection of embodiment 3 enzyme
[0085] The enzyme solution obtained above is used for the substrate-catalyzed reaction.
[0086] Coenzyme regeneration using glucose dehydrogenase BsGDH: Dissolve 1g of the substrate (compound I) in 40mL of 50mM pH7.0 sodium phosphate buffer containing 10% isopropanol, then add 800mg of glucose, stir and dissolve, then add 5mg of NADP+ , 250 mg ketoreductase LfHSDH lyophilized powder, 100 mg BsGDH lyophilized powder, and supplement the volume to 50 mL with buffer. The reaction solution was placed in a constant temperature water bath at 37°C, and stirred by magnetic force for reaction. During the reaction, the pH of the system was maintained at 7.0 with 1M sodium hydroxide solution. After reacting for 24 hours, samples were taken and detected by HPLC. The conversion rate of the substrate reached 62%, and the chiral purity value of the product was >99%.
[0087] Coenzyme regeneration using alcohol dehydrogenase ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com