Preparation methods for walnut-shell grafted beta-cyclodextrin type catalyst and 2-amino-3-cyano-4H-pyran derivative
A technology of pyran derivatives and walnut shells, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve problems such as unsatisfactory catalysts and long reaction times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The specific steps of this embodiment include two parts:
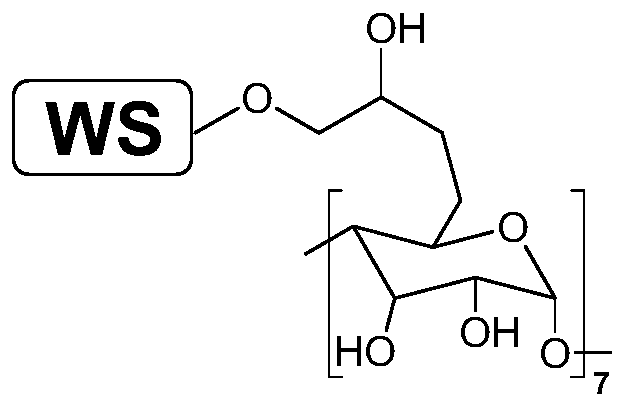
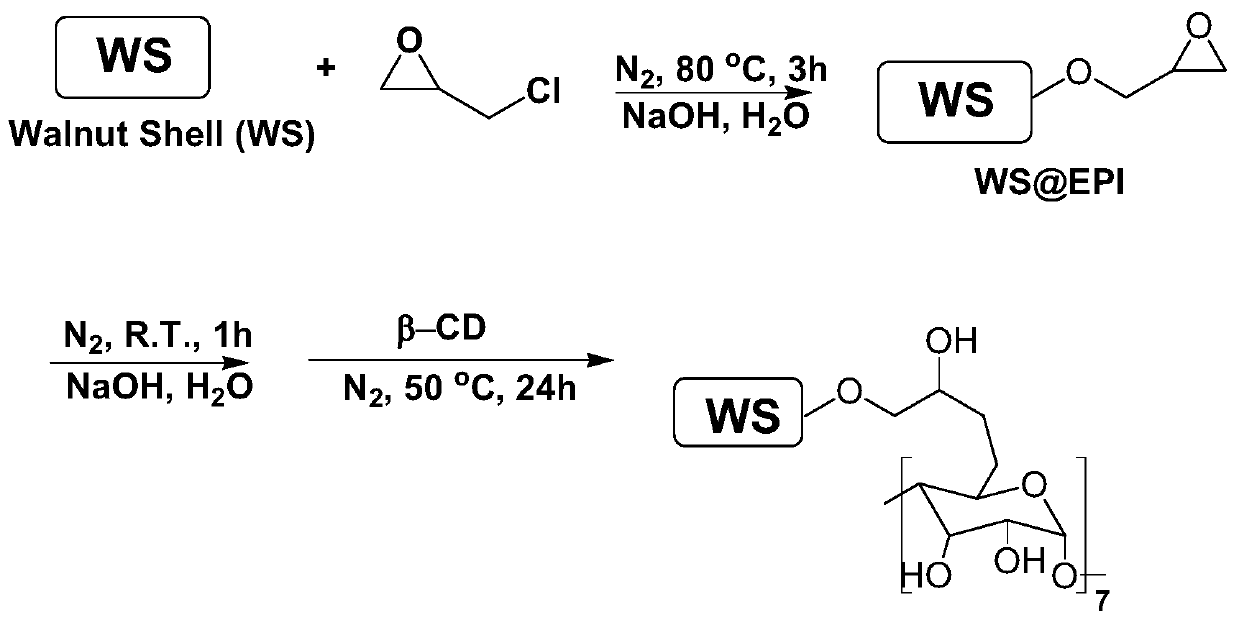
[0039] 1) Preparation of walnut shell grafted β-CD catalyst (WS@EPI / β-CD), the reaction route is as follows figure 2 :
[0040] 1) Put walnut shells, deionized water, sodium hydroxide and epichlorohydrin in the reaction bottle in turn, and then react for 3 hours in a nitrogen environment at 80°C; The volume dosage of sodium hydroxide is 4.8-5.0mL / g, the mass dosage of sodium hydroxide is 0.85-1g / g, and the volume dosage of epichlorohydrin is 1.8-2.0mL / g;
[0041] 2) After the reaction, the product in the reaction bottle was washed with water, centrifuged and dried to obtain epoxidized walnut shells, that is, WS@EPI;
[0042] 3) Add WS@EPI, deionized water and sodium hydroxide into the reaction bottle, and react at room temperature for 1 hour; among them, the volume of deionized water is 14-15mL / g based on the quality of WS@EPI , the mass dosage of sodium hydroxide is 2.8~3.0g / g;
[0043] 4) After the reacti...
Embodiment 2
[0050] The difference between Example 2 and Example 1 is: Step 2) is 2-amino-3-cyano-7,7-dimethyl-4-(4-chlorophenyl)-5-oxo-5,6 , the preparation of 7,8-4H-benzopyran (structural formula is as follows):
[0051]
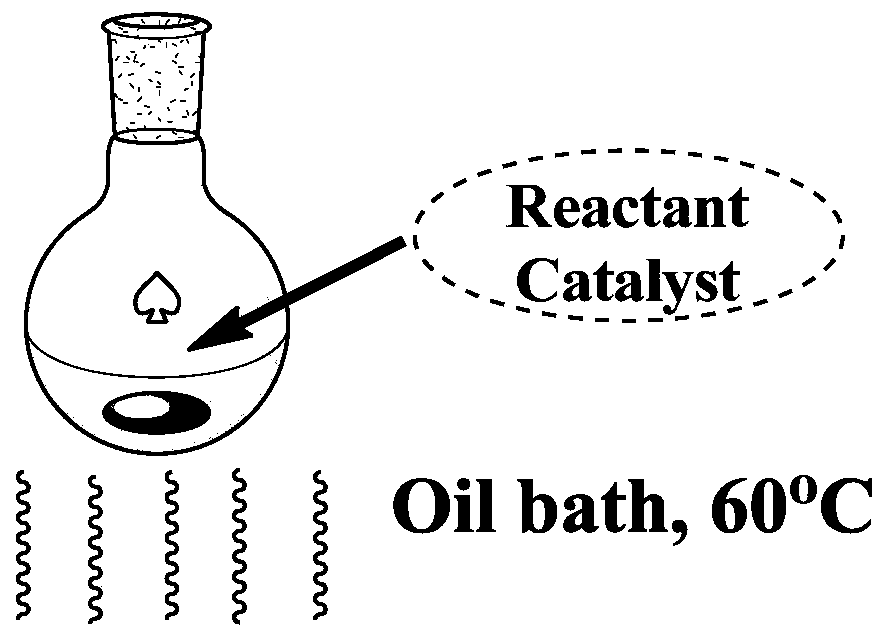
[0052] Add 0.002mol of p-chlorobenzaldehyde, malononitrile and dimedone into the reaction flask, then add 2mL of ethanol and 50mg of catalyst WS@EPI / β-CD to the reaction flask in sequence, and quickly place the reaction flask in React in an oil bath at 60°C for 5 minutes. After the reaction is over, add a certain amount of water to the reaction flask to quench the reaction, and filter to obtain a solid product containing the catalyst; then dissolve the solid product with ethanol, and perform multiple centrifugation operations to separate the product from the catalyst. Finally, the centrifuged solution was subjected to a rotary evaporation operation to obtain a crude product, which was then recrystallized and dried to obtain 0.64 g of the target product with a yiel...
Embodiment 3
[0055] The difference between Example 3 and Example 1 is: Step 2) is 2-amino-3-cyano-7,7-dimethyl-4-(4-methoxyphenyl)-5-oxo-5 , the preparation of 6,7,8-4H-benzopyran (structural formula is as follows):
[0056]
[0057] Add 0.002mol of p-methoxybenzaldehyde, malononitrile and dimedone into the reaction flask, then add 2mL of ethanol and 50mg of catalyst WS@EPI / β-CD to the reaction flask in sequence, and quickly put the reaction flask Placed in an oil bath at 60°C for 8 minutes. After the reaction is over, add a certain amount of water to the reaction flask to quench the reaction, and filter to obtain a solid product containing the catalyst; then dissolve the solid product with ethanol, and perform multiple centrifugation operations to separate the product from the catalyst. Finally, the centrifuged solution was subjected to a rotary evaporation operation to obtain a crude product, which was then recrystallized and dried to obtain 0.62 g of the target product with a yield ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap