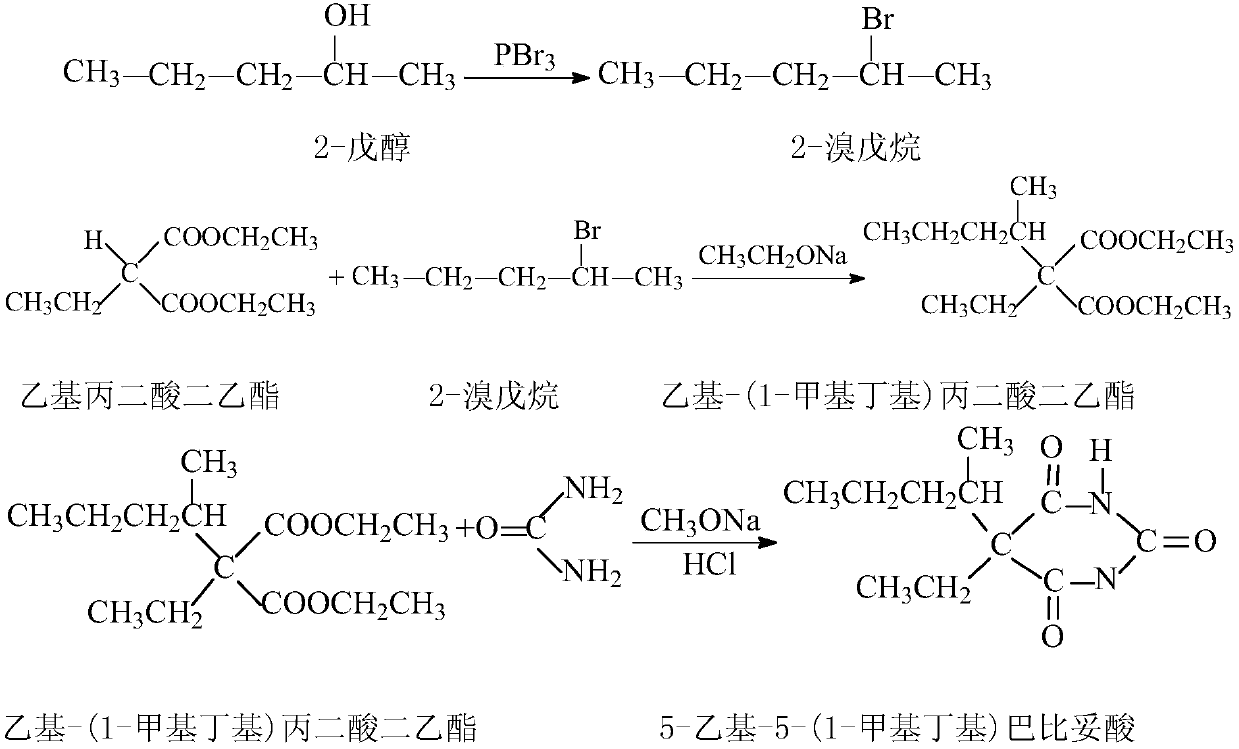
High-purity 5-ethyl-5-(1-methylbutyl)barbituric acid preparation method
A technology of methyl butyl and barbituric acid, applied in the direction of organic chemistry, can solve the problems of affecting product purity, incomplete reaction, and poor product appearance, and achieve the effects of solvent recycling, low production cost, and less waste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Step A: Add 700g of 2-pentanol and 18g of anhydrous sodium carbonate into a 2000L reaction bottle, stir and cool down to -10°C ~ -15°C, and slowly add 200g of phosphorus tribromide dropwise at a controlled temperature of -10±5°C. It takes about 2 hours to drip. It takes about 3 hours to control the reaction temperature from -10°C to room temperature under stirring, then gradually increase the temperature and distill until the internal temperature does not exceed 150°C, then stop the distillation. Evaporate the alkanol and dry it with anhydrous sodium sulfate, then add 20g of anhydrous sodium carbonate and stir for 5-10 minutes (the pH value of the alkanol solution should be neutral at this time), filter immediately, and the filtrate is subjected to atmospheric pressure fractionation, and the collection column The fraction with a top temperature of 110-113°C yielded 793.84 g of a mixture of 2-bromopentane and 2-pentanol. The content of 2-bromopentane was 33.74%.
[002...
Embodiment 2
[0026] Step A: Add 700g of 2-pentanol and 14g of anhydrous sodium carbonate into a 2000L reaction bottle, stir and cool down to -10°C ~ -15°C, and slowly add 175g of phosphorus tribromide dropwise at a temperature of -10±5°C. It takes about 2 hours to drip. It takes about 3 hours to control the reaction temperature from -10°C to room temperature under stirring, then gradually increase the temperature and distill until the internal temperature does not exceed 150°C, then stop the distillation. Evaporate the alkanol and dry it with anhydrous sodium sulfate, then add 20g of anhydrous sodium carbonate and stir for 5-10 minutes (the pH value of the alkanol solution should be neutral at this time), filter immediately, and the filtrate is subjected to normal pressure fractionation, and the collection column The fraction with a top temperature of 110-113°C yielded 756.65 g of a mixture of 2-bromopentane and 2-pentanol. The content of 2-bromopentane was 30.67%.
[0027] Step B: Add 3...
Embodiment 3
[0030] Step A: Add 700g of 2-pentanol and 42g of anhydrous sodium carbonate into a 2000L reaction bottle, stir and cool down to -10°C ~ -15°C, and slowly add 210g of phosphorus tribromide dropwise at a temperature of -10±5°C. It takes about 2 hours to drip. It takes about 3 hours to control the reaction temperature from -10°C to room temperature under stirring, then gradually increase the temperature and distill until the internal temperature does not exceed 150°C, then stop the distillation. Evaporate the alkanol and dry it with anhydrous sodium sulfate, then add 20g of anhydrous sodium carbonate and stir for 5-10 minutes (the pH value of the alkanol solution should be neutral at this time), filter immediately, and the filtrate is subjected to normal pressure fractionation, and the collection column The fraction with a top temperature of 110-113°C yielded 800.38 g of a mixture of 2-bromopentane and 2-pentanol. The content of 2-bromopentane was 34.98%.
[0031] Step B: Add 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com