M cell targeting and pH responding starch based carrier material and preparation method and applications thereof
A technology of cell targeting and carrier materials, which is applied in the direction of medical preparations, pharmaceutical formulas, and capsule delivery of non-active ingredients. Strong resistance, the effect of improving the transfer efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
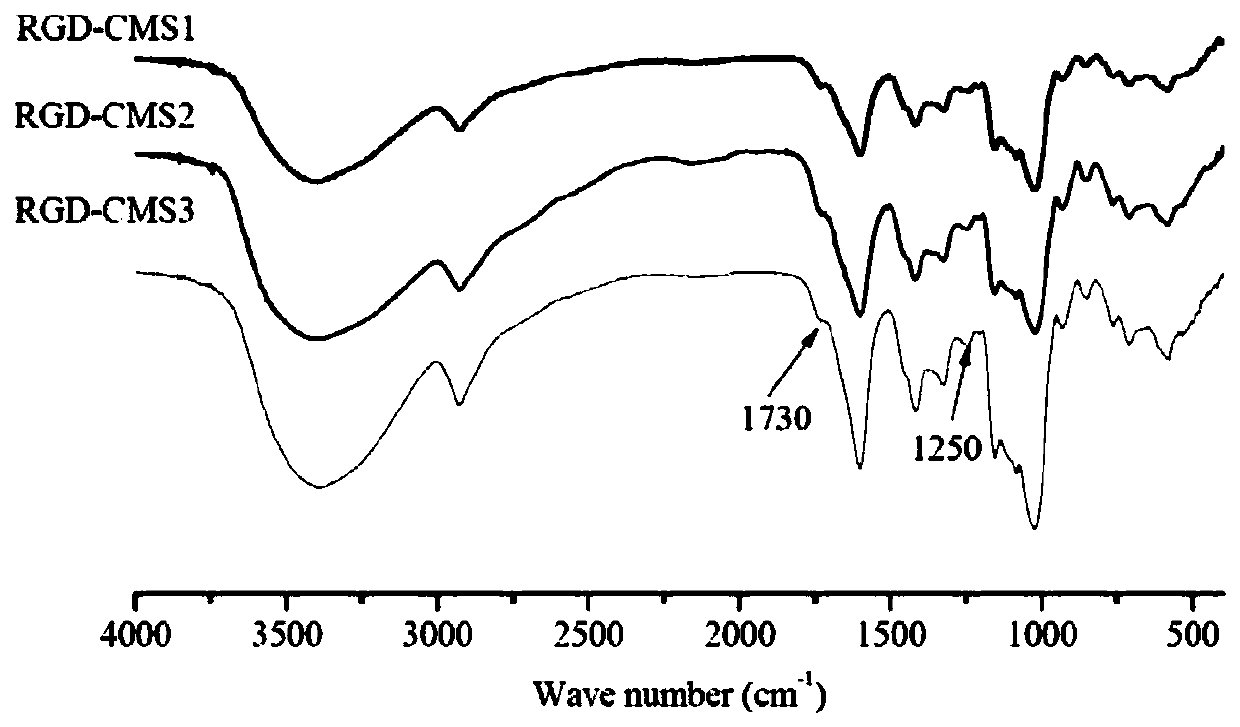
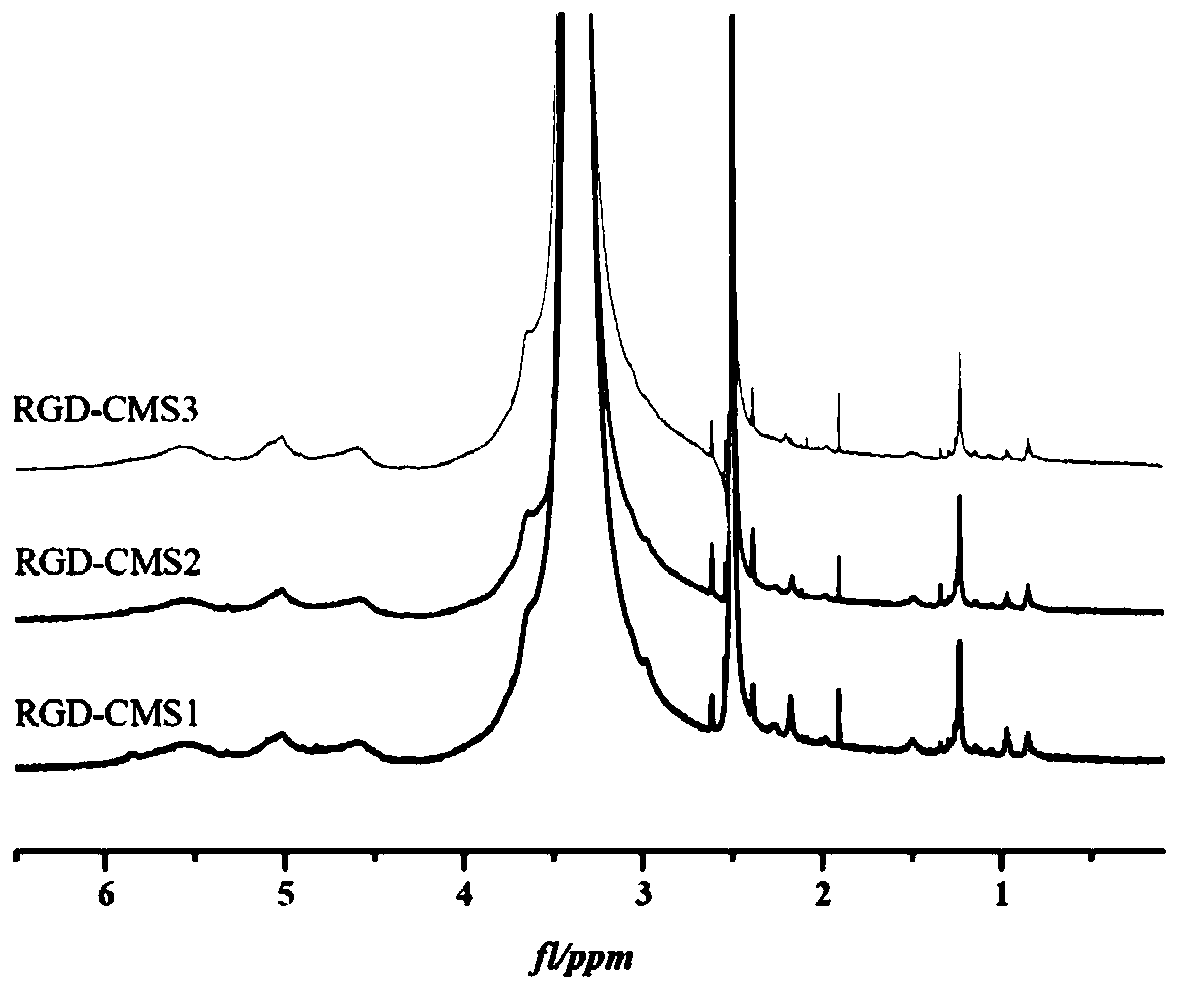
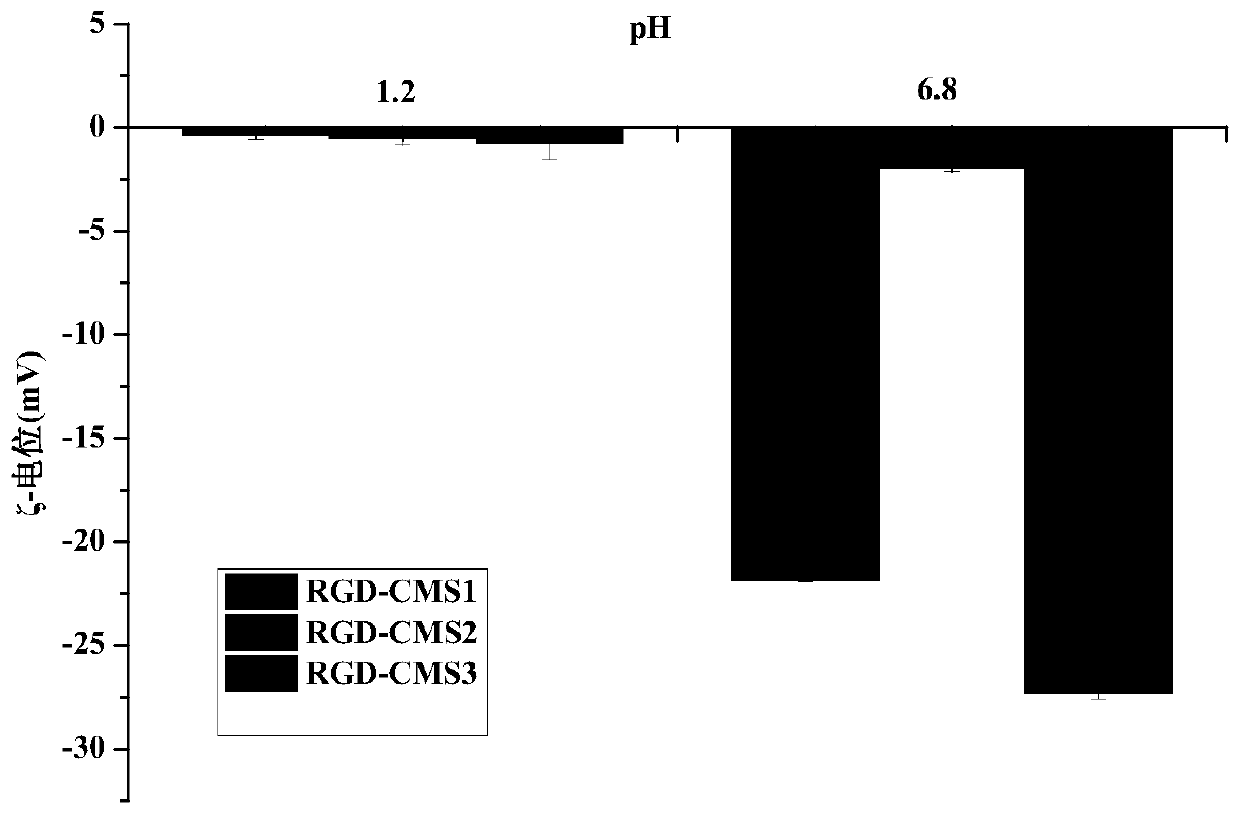
[0057] (1) According to monochloroacetic acid: native starch (molecular weight is 2.0×10 6 g / mol)=0.4 molar ratio to carry out etherification reaction, reaction temperature 50 ℃, reaction time 4h, the obtained carboxymethyl starch (CMS1) that carboxymethyl substitution degree is 0.27;
[0058] (2) According to molar ratio, carboxymethyl group: catalyst: GRGDS short peptide=1:1:1 ratio carries out acylation reaction, and reaction temperature is 35 ℃, and the reaction time is 24h, and wherein catalyzer is EDC and NHS (mole The ratio is 1:1), the concentration of carboxymethyl starch in the reaction system is 1% (w / v, g / mL), and the solvent is phosphate buffer (0.1M, pH=7.5). After the reaction was completed, it was dialyzed and freeze-dried to obtain a molecular weight of 2.11×10 6 g / mol, a starch-based carrier material (RGD-CMS1) with a carboxymethyl substitution degree of 0.27 and a targeting peptide GRGDS grafting amount of 1.12% (calculated based on N element content).
[...
Embodiment 2
[0065] (1) According to monochloroacetic acid: native starch (molecular weight is 1.0×10 6 g / mol)=0.1 molar ratio to carry out etherification reaction, reaction temperature 40 ℃, reaction time 2h, the carboxymethyl starch that makes carboxymethyl substitution degree is 0.04;
[0066] (2) The carboxymethyl starch with a carboxymethyl substitution degree of 0.04 prepared in step (1) was first enzymolyzed by pullulanase (unit enzyme activity 15U / g (carboxymethyl starch dry basis)) at 50°C for 24h Finally, use high temperature-resistant α-amylase (unit enzyme activity 100U / g (carboxymethyl starch dry basis)) to enzymolyze at 80°C for 30min to obtain a molecular weight of 6.99×10 4 Carboxymethyl starch (CMS2) of g / mol;
[0067] (3) carry out acylation reaction according to the molar ratio carboxymethyl group: catalyst: GRGDS short peptide=4:1:1 ratio, reaction temperature is 25 ℃, and the reaction time is 12h, and wherein catalyzer is EDC and NHS (molar ratio 1:1), the concentrat...
Embodiment 3
[0074] (1) According to monochloroacetic acid: native starch (molecular weight is 1.0×10 7 g / mol)=0.3 molar ratio to carry out etherification reaction, reaction temperature 45 ℃, reaction time 3h, the carboxymethyl starch that makes carboxymethyl substitution degree is 0.24;
[0075] (2) The carboxymethyl starch obtained in step (1) with a carboxymethyl substitution degree of 0.24 is enzymatically hydrolyzed at 50°C for 16 hours by pullulanase (unit enzyme activity 15U / g (carboxymethyl starch dry basis)) Finally, use high temperature resistant α-amylase (unit enzyme activity 15U / g (carboxymethyl starch dry basis)) to enzymolyze at 80°C for 10min to obtain a molecular weight of 4.56×10 5 Carboxymethyl starch (CMS3) of g / mol;
[0076] (3) The acylation reaction was carried out according to the molar ratio of carboxymethyl group:catalyst:GRGDS short peptide=2:1:1, the reaction temperature was 30°C, and the reaction time was 18h. Wherein the catalyst is EDC and NHS (the molar ra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com