(S)-1,3-thiazolyl phenyl furan thioformate compounds and preparation method and application thereof
A technology of thiazolylphenylfuranthiocarboxylic acid and ester compounds, which is applied in the field of preparation of heterocyclic compounds, to achieve the effects of reducing pathogenicity, reducing drug resistance, and delaying drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
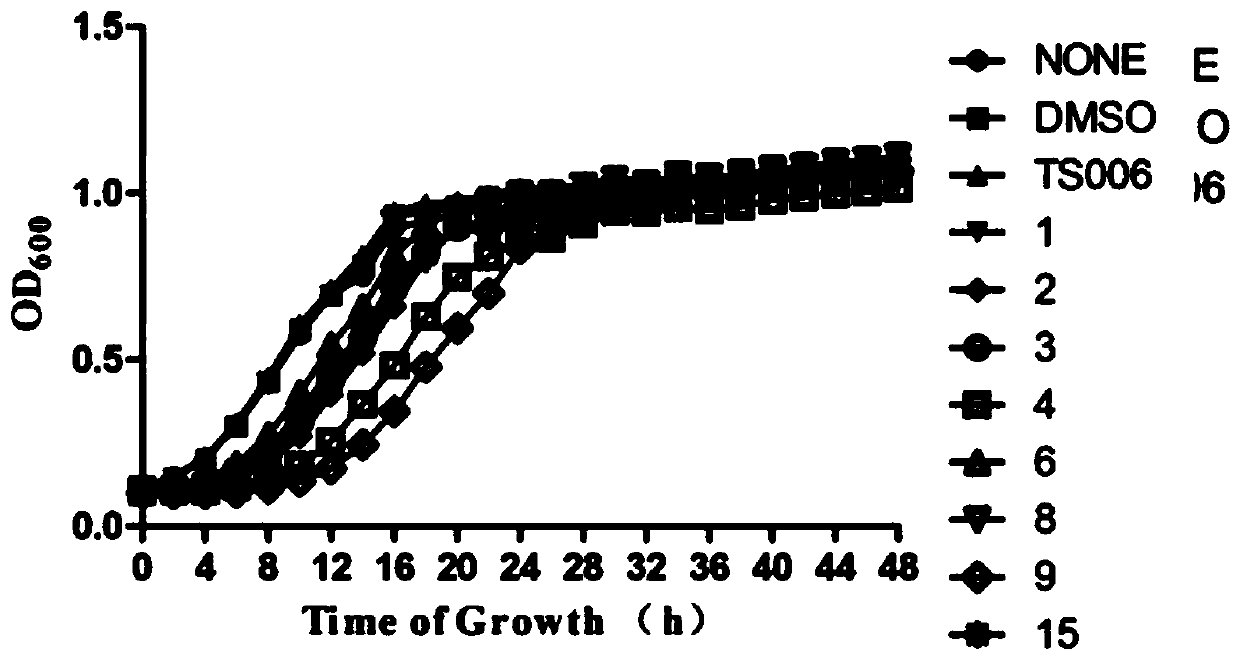
Image
Examples
Embodiment 1
[0035] Embodiment 1: the synthesis of compound III-1
[0036] The specific preparation process of compound III-1 is as follows:
[0037] Add 0.44g 5-(2-chlorophenyl)-2-furancarboxylic acid (2mmol) and 5mL thionyl chloride successively to a 50mL single-necked bottle; heat to reflux for 1.5 hours, stop heating, wait for the reaction solution to cool to room temperature, evaporate under reduced pressure Except the remaining thionyl chloride, the obtained furoyl chloride intermediate is directly used in the next step without purification;
[0038] In another 50 mL three-necked flask, 0.23 g (2.0 mmol) of mercaptothiazole, 0.55 g of potassium carbonate (4.0 mmol), and 20 mL of acetonitrile were sequentially added. Stir at room temperature for half an hour under nitrogen protection; cool to 0°C in an ice bath, slowly add a solution of furoyl chloride in acetonitrile (≈20 mL), and transfer to room temperature for reaction after the addition is complete. Thin-layer chromatography (T...
Embodiment 2
[0042] Embodiment 2: the synthesis of compound III-2
[0043] The method is the same as in Example 1, except that 5-(3-chlorophenyl)-2-furoyl chloride is used instead of 5-(2-chlorophenyl)-2-furoyl chloride to obtain pink solid compound III-2 .
[0044] 1 H NMR (400MHz, Chloroform-d) δ8.00(dd, J=7.9,1.6Hz,1H),7.97(d,J=3.3Hz,1H),7.60(d,J=3.3Hz,1H),7.50 (dd, J=8.0,1.2Hz,1H),7.44(d,J=3.8Hz,1H),7.41(td,J=7.7,1.3Hz,1H),7.35(dd,J=7.5,1.7Hz, 1H), 7.33(d, J=3.8Hz, 1H).
[0045]
Embodiment 3
[0046] Embodiment 3: the synthesis of compound III-3
[0047] The method is the same as in Example 1, except that 5-(4-chlorophenyl)-2-furoyl chloride is used instead of 5-(2-chlorophenyl)-2-furoyl chloride to obtain pink solid compound III-3 .
[0048] 1 H NMR (400MHz, Chloroform-d) δ7.97(d, J=3.3Hz, 1H), 7.77–7.73(m, 2H), 7.60(d, J=3.4Hz, 1H), 7.46–7.43(m, 2H), 7.42(d, J=3.8Hz, 1H), 6.84(d, J=3.8Hz, 1H).
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com