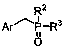
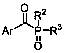Method for preparing arylphosphine oxide derivatives
A technology of aryl phosphine oxide and derivatives, applied in the field of preparation of organic compounds, can solve the problems of difficult operation, harsh reaction conditions, high toxicity of reagents, etc., and achieves simple reaction operation and post-treatment process, mild reaction conditions, and reaction time. short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of benzyl-diphenylphosphine oxide
[0038] Using potassium benzyl trifluoroborate and diphenyl phosphine oxide as raw materials, the reaction steps are as follows:
[0039] Add potassium benzyl trifluoroborate (99 mg, 0.5 mmol), diphenyl phosphine oxide (101 mg, 0.5 mmol), sodium persulfate (115 mg, 0.5 mmol), copper sulfate (16 mg, 0.5 mmol) into the reaction flask 0.1 mmol) and methanol (5 mL), react at room temperature;
[0040] TLC tracks the reaction until it is completely over;
[0041] After the reaction, the crude product obtained was separated by column chromatography (dichloromethane:methanol = 95:5) to obtain the target product (yield 75%). The analytical data of the product is as follows: 1 H NMR (400 MHz, DMSO-d 6 ) δ 7.85–7.77 (m, 4H), 7.60–7.44 (m, 6H), 7.28–7.15 (m, 5H), 6.48 (dd, J = 17.8, 5.8 Hz, 1H), 5.62(t, J = 6.4 Hz, 1H).
Embodiment 2
[0042] Example 2: Synthesis of (2-chlorobenzyl)-bis(4-methoxyphenyl)phosphine oxide
[0043] Using (2-chlorobenzyl) potassium trifluoroborate and bis(4-methoxyphenyl) phosphine oxide as raw materials, the reaction steps are as follows:
[0044] Add (2-chlorobenzyl) potassium trifluoroborate (116 mg, 0.5 mmol), bis(4-methoxyphenyl) phosphine oxide (262 mg, 1 mmol), potassium persulfate (270 mg, 1.0 mmol), cuprous chloride (5 mg, 0.05 mmol) and ethanol (5 mL), 40 o C reaction;
[0045] TLC tracks the reaction until it is completely over;
[0046] After the reaction, the crude product obtained was separated by column chromatography (dichloromethane:methanol = 95:5) to obtain the target product (yield 82%). The analytical data of the product is as follows: 1 H NMR (400 MHz, DMSO-d 6 ) δ 7.89–7.80 (m, 4H), 7.64–7.48 (m, 4H), 7.32–7.19 (m, 4H), 6.50 (dd, J = 17.8, 5.8 Hz, 1H), 5.68(t, J = 6.4 Hz, 1H), 3.67 (s, 6H).
Embodiment 3
[0047] Example 3: Synthesis of (3-chlorobenzyl)-bis(4-tolyl)phosphine oxide
[0048] Using (3-chlorobenzyl) potassium trifluoroborate and bis(4-methylphenyl)phosphine oxide as raw materials, the reaction steps are as follows:
[0049] Add (3-chlorobenzyl) potassium trifluoroborate (116 mg, 0.5 mmol), bis(4-methylphenyl) phosphine oxide (345 mg, 1.5 mmol), and ammonium persulfate (342 mg) to the reaction flask , 1.5 mmol), cuprous iodide (22.8 mg, 0.12 mmol) and acetonitrile / water (2.5 mL: 2.5 mL), 50 o C reaction;
[0050] TLC tracks the reaction until it is completely over;
[0051] After the reaction, the crude product obtained was separated by column chromatography (dichloromethane: methanol = 95:5) to obtain the target product (yield 85%). The analytical data of the product is as follows: 1 H NMR (400 MHz, DMSO-d 6 ) δ 7.88-7.81 (m, 4H), 7.65-7.47 (m, 4H), 7.31-7.18 (m, 4H), 6.51 (dd, J = 17.8, 5.8 Hz, 1H), 5.66(t, J = 6.4 Hz, 1H), 2.21 (s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com