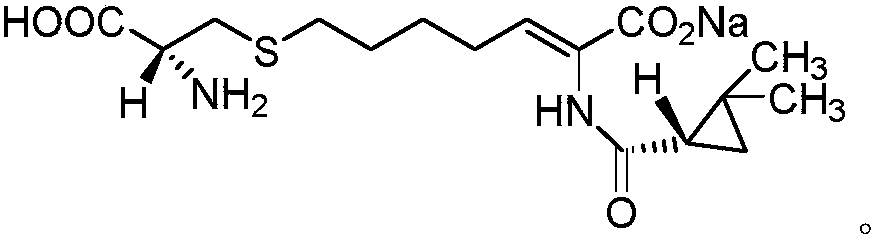
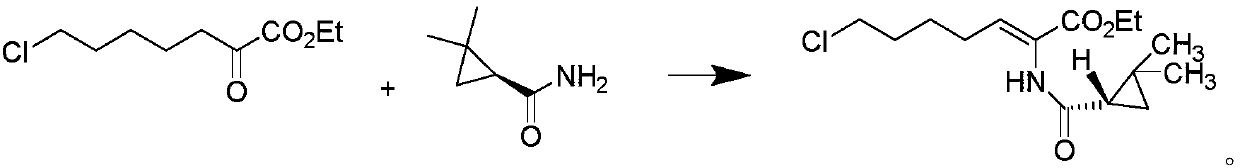Method for purifying intermediate of cilastatin sodium
A technology of cilastatin sodium and a purification method, which is applied in the field of drug synthesis, can solve the problems of large amount of solvent, cumbersome operation, and long operation time, and achieve the effects of cheap and easy-to-obtain reagents, simplified operation process, and reduced possibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 10.0 g of (E-Z)-7-chloro-2((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid ethyl ester crude product (HPLC purity 85%, E configuration Impurity 12%), add 10ml of acetone, stir, then add 10ml of ethanol, dropwise add 50ml of purified water, after the dropwise addition is completed, use hydrochloric acid with a mass fraction of 1% to adjust pH4, then cool down to 5°C, keep stirring for 3h, and suction filter, The obtained solid 8.4g is Z-7-chloro-2((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid ethyl ester, HPLC purity 99.5%, E configuration impurity 0.06%.
Embodiment 2
[0031] Weigh 10.0 g of (E-Z)-7-chloro-2((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid ethyl ester crude product (HPLC purity 85%, E configuration impurity 12%), add 20ml of acetone, stir, then add 10ml of isopropanol, dropwise add 50ml of purified water, after the dropwise addition, use 5% acetic acid to adjust the pH to 6, then cool down to 10°C, keep stirring for 3h, pump Filter to obtain 8.2 g of solid Z-7-chloro-2 ((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid ethyl ester, HPLC purity 99.1%, E structure Type impurity 0.09%.
Embodiment 3
[0033] Weigh 10.0 g of (E-Z)-7-chloro-2((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid ethyl ester crude product (HPLC purity 85%, E configuration impurity 12%), add 15ml of acetone, stir, then add 20ml of tert-butanol, dropwise add 80ml of purified water, adjust the pH5 with sulfuric acid with a mass fraction of 1% after the dropwise addition, then cool down to 0°C, keep stirring for 3h, pump Filter to obtain 8.5 g of solid, which is Z-7-chloro-2 ((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid ethyl ester, HPLC purity 99.0%, E structure Type impurities 0.10%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com