Enhanced anti-tumor fusion protein, preparation method and use thereof
A fusion protein and anti-tumor technology, which is applied in the field of fusion protein and preparation, to achieve obvious killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Construction of vectors expressing various proteins
[0037] The expression plasmid adopted is pET22b (Novagen Company), which contains various restriction enzyme cutting sites (BamHI-EcoRI-SacI-SalI-HindIII-NotI-XhoI) for the insertion of DNA fragments encoding foreign proteins, and outside expression The C-terminus of the source protein has a His-tag (containing 6 His) for protein purification.
[0038] DNA fragments were synthesized by TAKARA Company and Shanghai Sangon Bioengineering Company.
[0039] The nucleic acid sequence of synthetic B7.1 is shown by SEQ ID No.1;
[0040] The nucleic acid sequence of synthetic B7.2 is shown by SEQ ID No.3;
[0041] The nucleic acid sequence of the synthetic SEA is shown in SEQ ID No.5;
[0042] The nucleic acid sequence of the synthetic B7.1-B7.2 fusion protein is shown in SEQ ID No.7, and there is a nucleic acid fragment (gtcgacaagctttccggcggaggtggc) (shown in SEQ ID No.15) of a connecting peptide between B7.1 an...
Embodiment 2
[0057] Example 2 Expression, denaturation and renaturation and purification of various proteins
[0058] Various expression plasmids pET22b-B7.1, pET22b-B7.2, pET22b-SEA, pET22b-B7.1-B7.2, pET22b-B7.1-SEA, pET22b-B7.2-SEA and pET22b-B7 .1-B7.2-SEA were transformed into Escherichia coli BL21(DE3) by electroporation, and the positive bacteria were screened by antibiotic Amp (Ampicillin). Next, the process of expression, denaturation, renaturation and purification of various proteins is roughly the same, and the operation is as follows:
[0059] Escherichia coli BL21(DE3) containing the expression plasmid was first cultured at 37°C on a large scale, and then IPTG (Isopropylthio-β-D-galactoside) was added to make the concentration reach 1mM and cultured at 30°C overnight to induce protein expression. On the second day, the culture medium was centrifuged and the bacteria were collected, the cell wall was broken by ultrasonic method, and the inclusion body precipitate was collected...
Embodiment 3
[0060] Embodiment 3 tumor suppression experiment
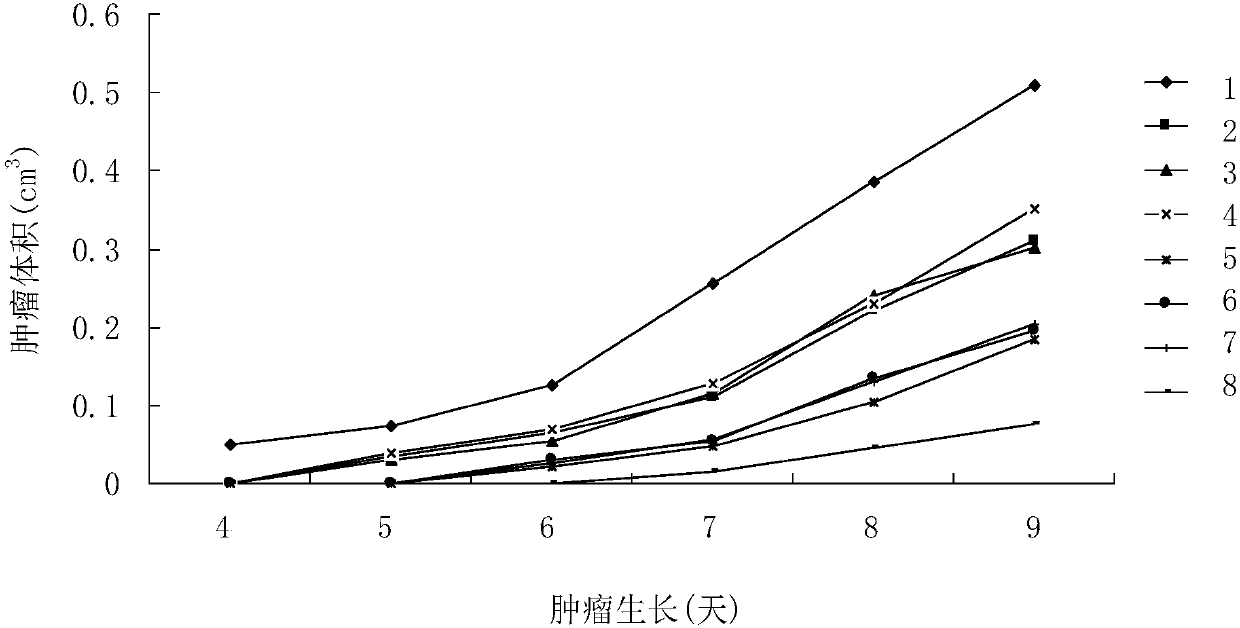
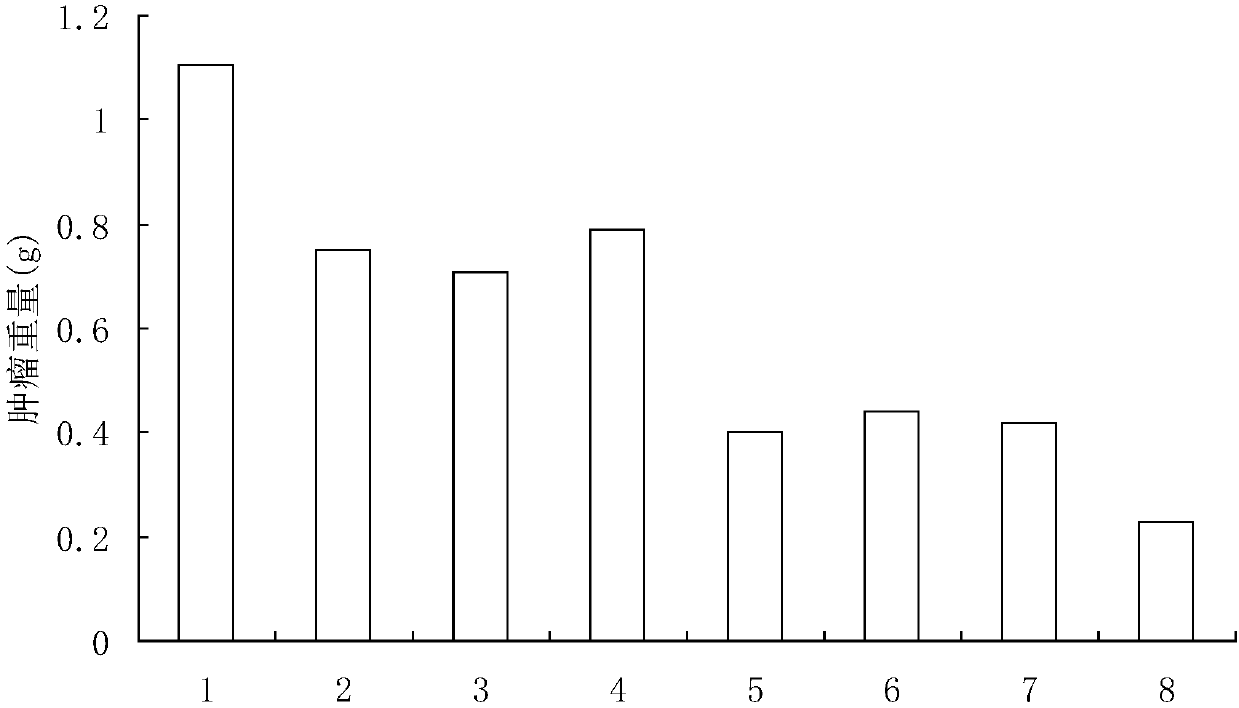
[0061] Select male ICR mice, 4-5 weeks old, 18-22g, and divide them into 8 groups randomly, 30 mice in each group. Mouse sarcoma cells S180 were cultured from mouse ascites, and 5x10 5 Mouse sarcoma cells S180 were inoculated in the right armpit of the mice, and then B7.1, B7.2, SEA, B7.1-B7.2, B7.1- SEA, B7.2-SEA and B7.1-B7.2-SEA protein solution 0.2ml (500pmol) / only, and control group injected equal amount of normal saline (0.9%NaCl), the 9th day put to death mice, observed small The tumor growth of the mice and the weight of the tumors after the mice were sacrificed were measured.
[0062] figure 1 Indicates that various proteins inhibit tumor growth, the horizontal axis in the figure represents the growth days of tumor-bearing mice, and the vertical axis represents the tumor volume (cm 3 ), the number of the right curve in the figure indicates the grouping of the experimental mice, 1 is the normal saline group, 2 is t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com