Aβ42 modified protein with the function of preventing protein aggregation and its expression and purification method
A protein and expression vector technology, applied in the fields of biotechnology and genetic engineering, can solve problems such as aggregation characteristics and toxicity research, and achieve the effects of high yield, high expression efficiency and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1: pET22b-His 6 -Aβ42 expression vector plasmid and recombinant His 6 - Construction of Aβ42 Escherichia coli expression strain
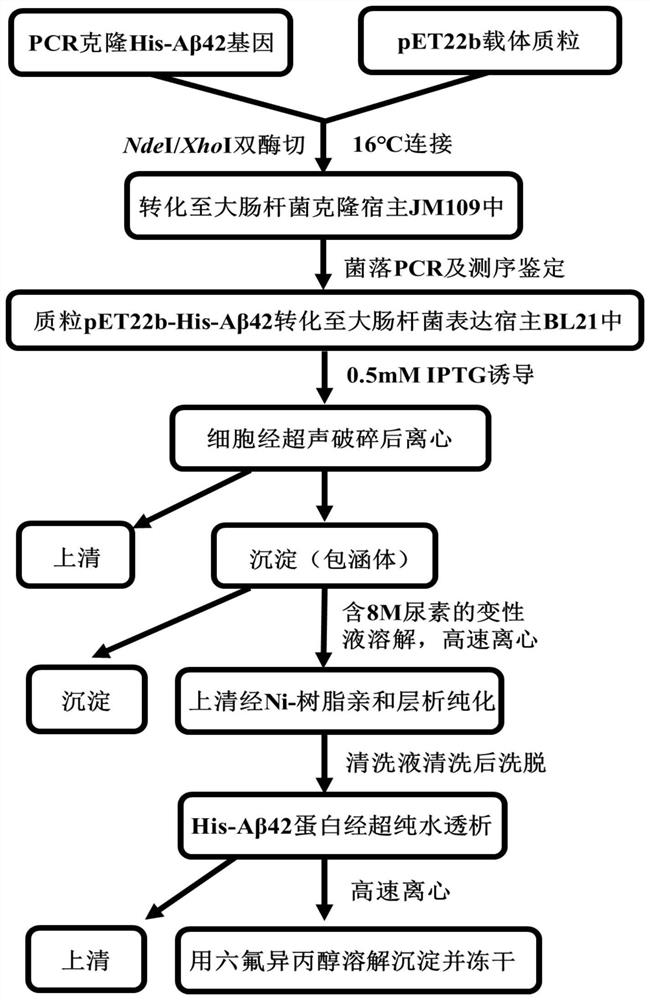
[0078] His 6 - Aβ42 expression and purification flow chart as shown figure 1 shown;
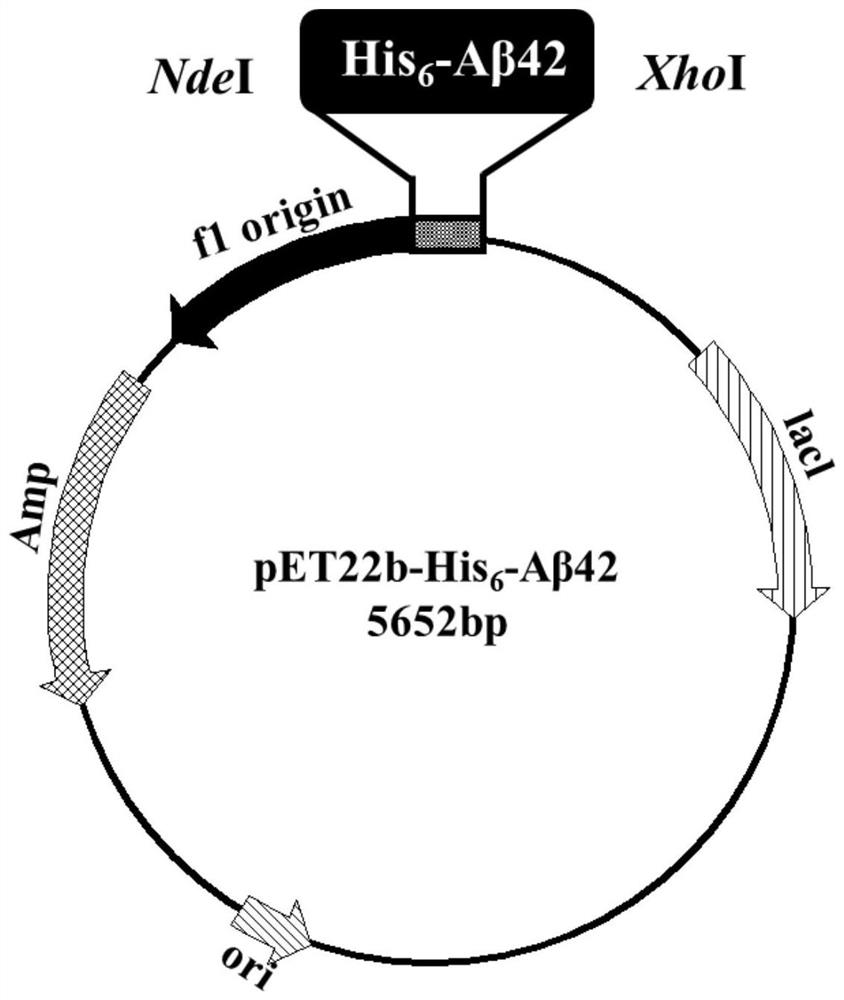
[0079] Expression vector pET22b-His 6 -Aβ42 construction schematic diagram as shown in figure 2 shown;
[0080] (1) Perform codon optimization on the Aβ42 gene sequence according to the amino acid sequence of Aβ42 and the codon preference of Escherichia coli to obtain the amino acid sequence of Aβ42 such as SEQ ID No.3 and the nucleotide sequence such as SEQ ID No.4;
[0081] (2) Using primer NdeⅠ-His 6 -Aβ42-F and XhoⅠ-His 6 -Aβ42-R, the primer sequences are shown in SEQ ID No.5 and SEQ ID No.6. The upstream primer NdeⅠ-His 6 -Aβ42-F contains His 6 The gene sequence of the tag, add His at the upstream of the Aβ42 gene sequence by PCR 6 tag, get His 6 -Aβ42 target gene sequence as shown in SEQ ID No.2, His 6 - The amino acid sequence o...
Embodiment 2
[0096] Example 2: His 6 -Expression and purification of Aβ42 protein
[0097] His 6- Aβ42 expression and purification flow chart as shown figure 1 shown.
[0098] (1)His 6 -Aβ42 protein expression: use 1mM isopropyl-β-D-thiogalactopyranoside to induce protein expression, and the engineering bacteria BL21-His obtained in Example 1 6 -Aβ42 was induced at 30°C for 20 hours. After the induction, the bacterial liquid was centrifuged to collect the bacterial cells to obtain 6 -Escherichia coli thalline of Aβ42 fusion protein;
[0099] (2) His 6 -Purification of Aβ42 protein: Resuspend the bacteria obtained above with buffer, add 1% phenylmethylsulfonyl fluoride, sonicate after 30 minutes in ice bath, and centrifuge at 12000 rpm for 30 minutes. 6 -Aβ42 protein is mainly expressed in the form of inclusion bodies, and the inclusion bodies are denatured and dissolved by using buffer A solution containing urea.
[0100] The composition of the denaturing solution (buffer A) is: 20...
Embodiment 3
[0106] Example 3: His 6 -Aβ42 peptide identification
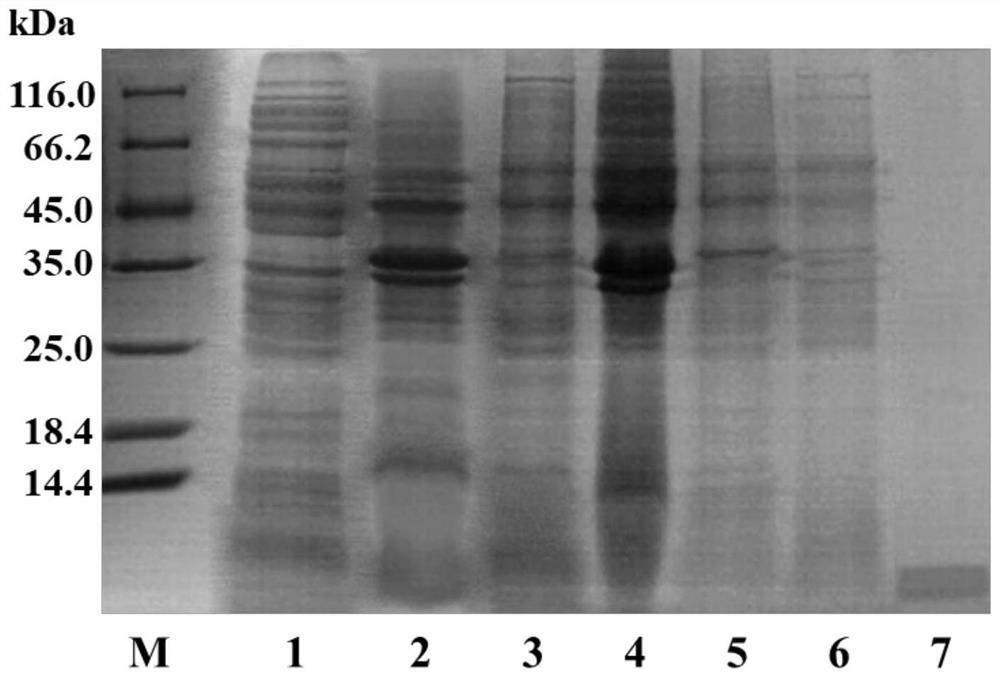
[0107] (1) Electrophoretic identification: the His prepared in Example 2 of the present invention 6 - The purity and molecular weight of Aβ42 protein were determined by SDS-PAGE electrophoresis, such as image 3 As shown, there is an obvious band around 5.47kDa. The protein concentration was detected by the NanoDrop 2000 protein and nucleic acid quantitator, and the final calculation of 1L bacterial solution can obtain about 22mg His 6 - Aβ42 polypeptide.
[0108] (2) Mass spectrometry identification: the purified His 6 -Aβ42 polypeptide was identified by MALDI TOF mass spectrometry, such as Figure 4 As shown, the peak with a charge-to-mass ratio of 5470 is His 6 -Aβ42 polypeptide, so it can be proved that this protein is the target protein His 6 - Aβ42.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com