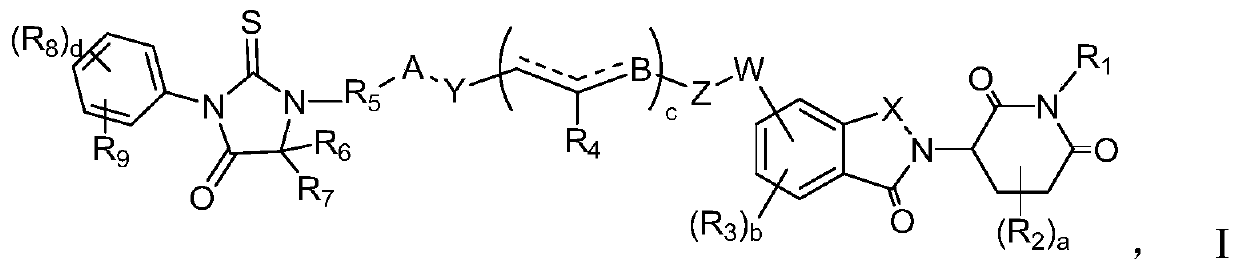
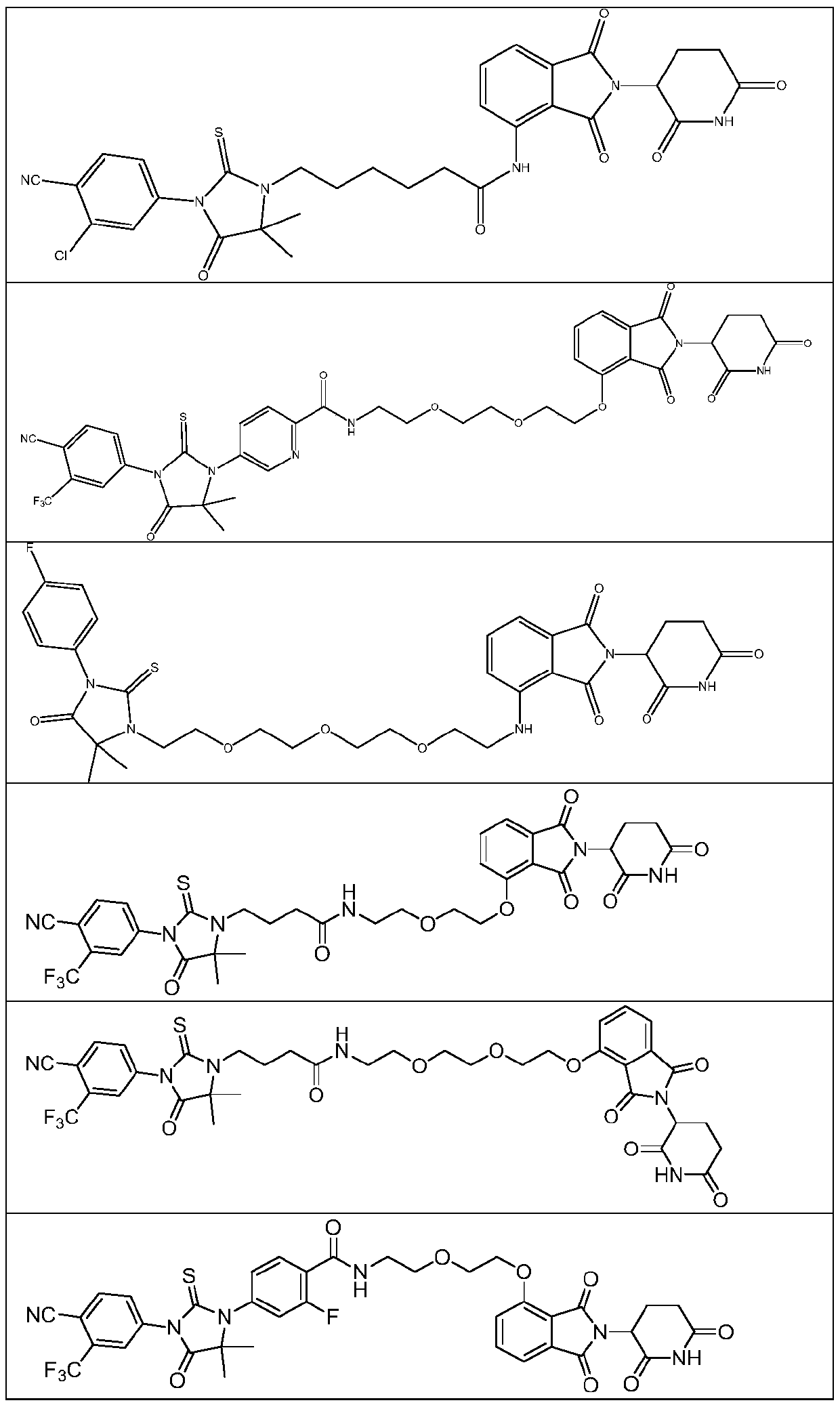
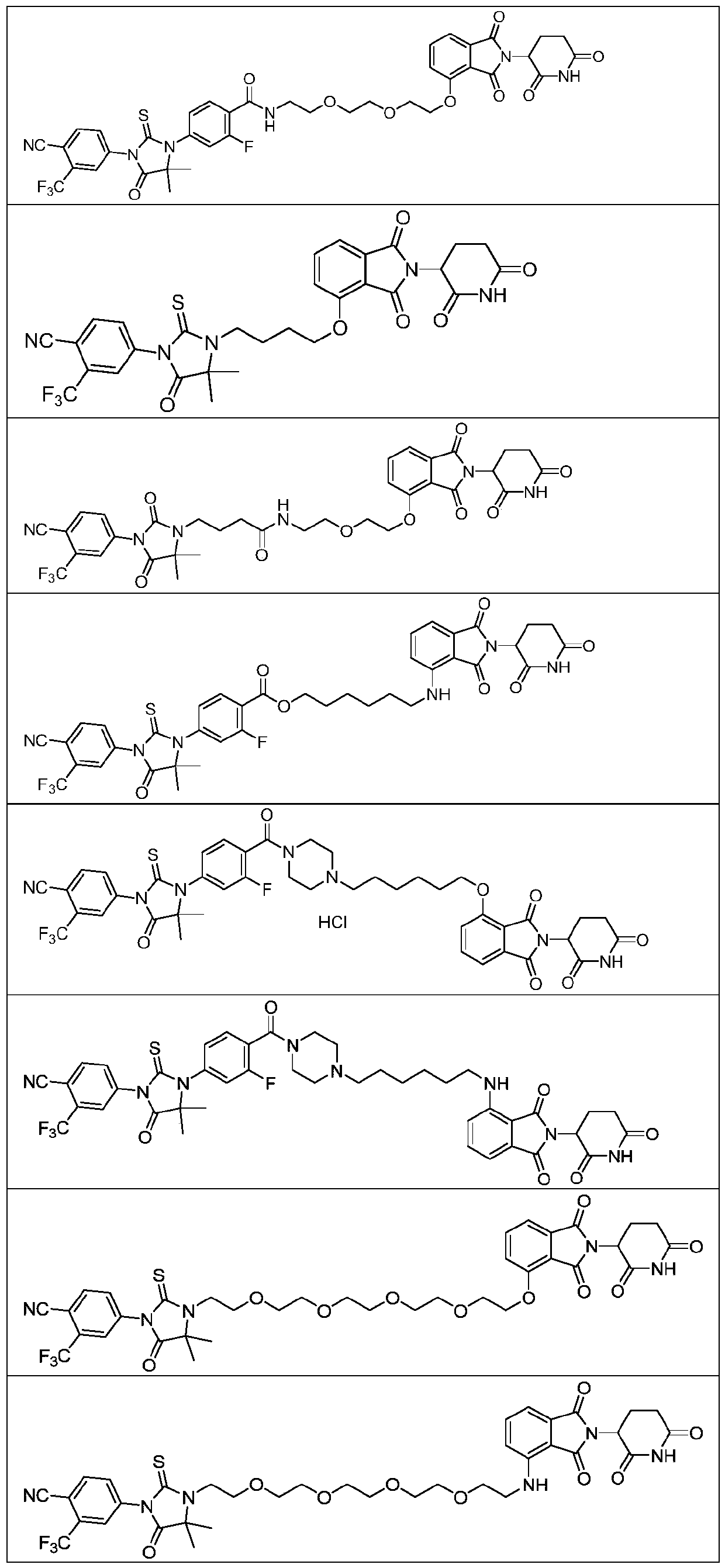
Compound with androgen receptor degradation activity
A technology of compounds and substituents, applied in the field of new compounds and their ability to degrade androgen receptors, can solve problems such as the inability to prevent the development of prostate cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0145] Compound preparation
[0146] Preparation
[0147] The preparation method of the compound of formula I of the present invention is described in more detail below, but these specific methods do not constitute any limitation to the present invention. The compound of the present invention can also be conveniently prepared by optionally combining various synthetic methods described in the specification or known in the art. Such a combination can be easily performed by those skilled in the art to which the present invention belongs.
[0148] The following reaction schemes illustrate the preparation of compounds of the invention. Unless otherwise indicated, A, B, W, Y, Z, X, a, b, c, d, R in the reaction schemes and ensuing discussions 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 is as defined above; M1 is a leaving group or can be converted into a leaving group, such as halogen, sulfonate, alcohol, aldehyde, acid.
[0149] In general, compounds of formula I c...
Embodiment 1
[0188]
[0189] Synthesis of compound 3
[0190] Methyl 6-aminocaproate (1.45 g) and acetone cyanohydrin (0.85 g) were dissolved in methanol (20 ml), and stirred overnight at room temperature. The reaction solution was concentrated to obtain the crude intermediate 1. The crude intermediate 1 was completely dissolved in anhydrous tetrahydrofuran (20ml), 3-chloro-4-cyanobenzene isothiocyanate (2.00g) and triethylamine (2.02g) were added, and the reaction solution was refluxed for 2h. The reaction solution was concentrated, the residue was dissolved in water (20ml) and ethanol (20ml), hydrochloric acid (20ml) was added, refluxed for 1h, the reaction solution was cooled, the organic solvent was removed under reduced pressure, and the aqueous phase was adjusted to pH 8 with saturated sodium bicarbonate -9, extracted with ethyl acetate (20ml*3), the organic phases were combined, concentrated under reduced pressure, and the residue was subjected to silica gel column chromatograph...
Embodiment 2
[0196]
[0197] Synthesis of compound 6
[0198] Under nitrogen protection, 2-amino-isobutyric acid methyl ester (1.40g), 5-bromopyridine-2-carboxylic acid (2.02g) 1,8-diazabicycloundec-7-ene (3.04 g), cuprous iodide (381mg) was added to N,N-dimethylacetamide (50ml), and heated at 150°C for 4h. The reaction solution was concentrated under reduced pressure, and the residue was purified with a silica gel column to obtain compound 6 (613 mg). MS(ESI):239[M+H] + .
[0199] Synthesis of compound 7
[0200] Under the protection of nitrogen, compound 6 (610mg), 3-trifluoromethyl-4-cyanobenzene isothiocyanate (584mg) and triethylamine (517mg) were dissolved in anhydrous tetrahydrofuran (20ml), and reflux reaction 2h. The reaction solution was concentrated under reduced pressure, and the residue was purified with a silica gel column to obtain compound 7 (835 mg). MS(ESI):435[M+H] + .
[0201] Synthesis of Compound 8
[0202] Compound 7 (434mg), 2-(2-(2-aminoethoxy)ethoxy)et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com