NASICON type solid electrolyte with high ionic conductivity and preparation method thereof
A solid electrolyte and ionic conductivity technology, applied in electrolytes, circuits, electrical components, etc., can solve the problems of low ionic conductivity at room temperature, reduced lithium battery performance, poor chemical stability, etc., to reduce grain boundary resistance and increase compactness , The effect of improving the conductivity at room temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
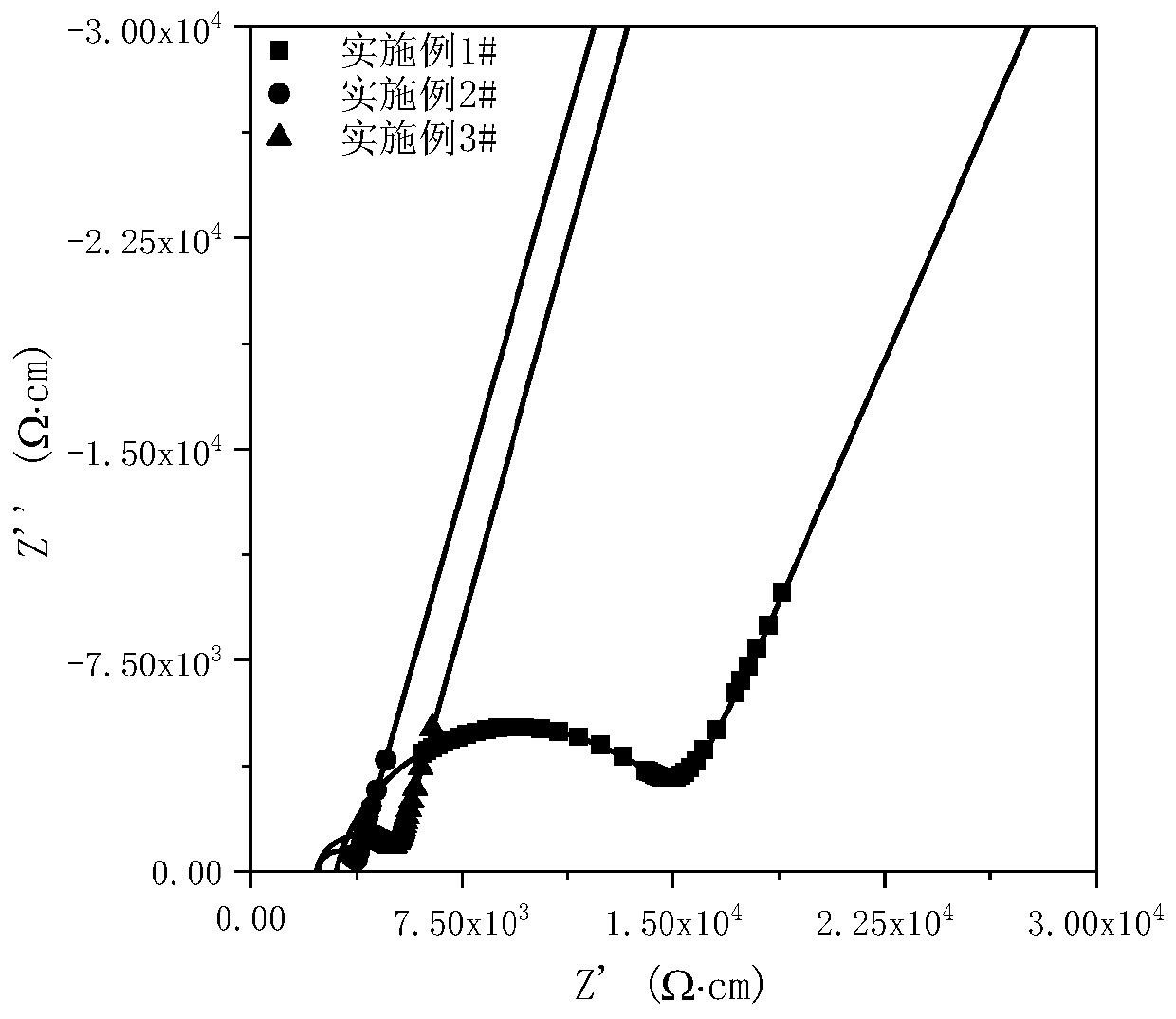
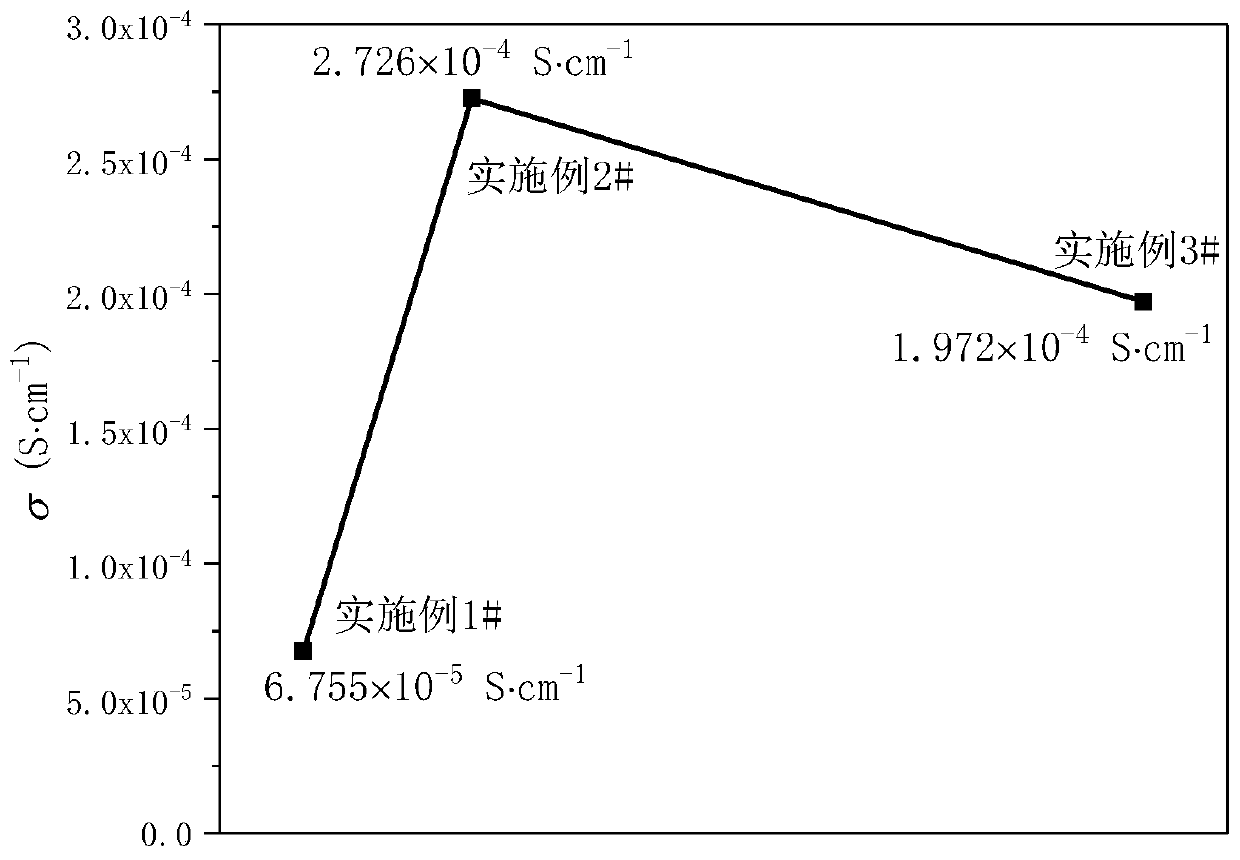
Embodiment 1
[0028] The raw material composition is shown in Table 1, and the specific preparation process is as follows:
[0029] Weigh raw materials (20g in total):
[0030]
[0031] Put the weighed raw materials in an agate mortar and grind them fully to form a mixed powder, put the ground mixed powder in a platinum crucible, and put it in a muffle furnace at about 200°C, preheat for 2 to 24 hours to remove impurities and moisture. The mixture obtained after preheating was placed in an agate mortar and fully ground again to obtain a uniformly dry powder, and then the prepared powder was placed in a platinum crucible again and melted in a high-temperature furnace over 1100°C for 10 minutes, and then The temperature was raised to not lower than 1200°C and kept for about 60 minutes. Thereafter, lower the temperature to about 1100°C and let stand for about 30 minutes. Cast the uniform glass liquid on the preheated cast iron mold, and transfer it to the annealing furnace quickly after ...
Embodiment 2
[0033] The raw material composition is shown in Table 1, and the specific preparation process is as follows:
[0034] Weigh raw materials (20g in total):
[0035]
[0036] Put the weighed raw materials in an agate mortar and grind them fully to form a mixed powder, put the ground mixed powder in a platinum crucible, and put it in a muffle furnace at about 200°C, preheat for 2 to 24 hours to remove impurities and moisture. The mixture obtained after preheating was placed in an agate mortar and fully ground again to obtain a uniformly dry powder, and then the prepared powder was placed in a platinum crucible again and melted in a high-temperature furnace over 1100°C for 10 minutes, and then The temperature was raised to not lower than 1200°C and kept for about 60 minutes. Thereafter, lower the temperature to about 1100°C and let stand for about 30 minutes. Cast the uniform glass liquid on the preheated cast iron mold, and transfer it to the annealing furnace quickly after ...
Embodiment 3
[0038] The raw material composition is shown in Table 1, and the specific preparation process is as follows:
[0039] Weigh raw materials (20g in total):
[0040]
[0041] Put the weighed raw materials in an agate mortar and grind them fully to form a mixed powder, put the ground mixed powder in a platinum crucible, and put it in a muffle furnace at about 200°C, preheat for 2 to 24 hours to remove impurities and moisture. The mixture obtained after preheating was placed in an agate mortar and fully ground again to obtain a uniformly dry powder, and then the prepared powder was placed in a platinum crucible again and melted in a high-temperature furnace over 1100°C for 10 minutes, and then The temperature was raised to not lower than 1200°C and kept for about 60 minutes. Thereafter, lower the temperature to about 1100°C and let stand for about 30 minutes. Cast the uniform glass liquid on the preheated cast iron mold, and transfer it to the annealing furnace quickly after it...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com